Blood coagulation
Molecular switches guide blood forming cells
Scientists from the University of Würzburg successfully elucidated new details about a circuit regulating platelet biogenesis. These important findings could contribute to a better understanding of the mechanism leading to bleeding disorders.
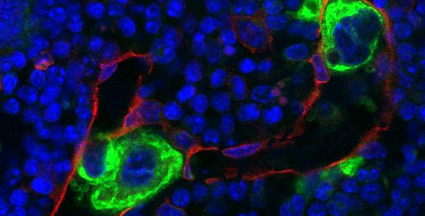
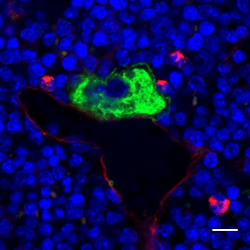
Platelets play a crucial role in hemostasis. At sites of vascular damage they attach to the subendothelial matrix, form a plug that seals the wound and contribute to tissue repair. Due to their short lifespan, new platelets need to be constantly generated. To this end, giant precursor cells in the bone marrow, so-called megakaryocytes, undergo a complex maturation process and finally release platelets into the bloodstream. Defects in platelet biogenesis can result in dramatically decreased numbers or malfunctioning of circulating platelets, thus affecting hemostasis in patients. Unfortunately, the detailed mechanisms regulating megakaryocyte maturation and platelet biogenesis still remain elusive.
Modifications cause drastic consequences
Researchers from the Rudolf Virchow Center for Experimental Biomedicine and the University Hospital Würzburg now succeeded in identifying a decisive regulatory circuit in platelet biogenesis. The group of Prof. Bernhard Nieswandt was able to decipher vital regulating factors in megakaryocytes. Small proteins, the Rho-GTPases, serve as molecular switches in the regulation of important cellular functions such as maturation, as well as orientation towards the blood vessels. Thus, the RHo-GTPases allow megakaryocytes to properly produce platelets. ´We could show that the complete absence or even a defect of these switches disrupts the orientation of the megakaryocyte, which then transmigrate through the blood vessel.´ says Prof. Nieswandt, director of the study. Indeed, under these conditions normal platelet biogenesis is abolished, leading to a drastic decrease in platelet count and bleeding complications in mice. These astonishing findings built the basis for a new understanding of platelet generation in bone marrow and most likely also provide new insights into the development of other blood cells.
Therapeutical approach in bleeding disorders
The discovery of this Rho-GTPase-dependent regulatory circuit in platelet biogenesis encourages the scientists to gain new insights in bleeding disorders, such as the Bernard-Soulier Syndrome. Patients suffering from this disorder endure a severe reduction of the platelet count, which is accompanied by a life-long profound bleeding complication. ´Our results open the way for new therapeutic approaches to treat diseases which are connected to defective platelet biogenesis.´ hopes Prof. Nieswandt.
Source: Rudolf-Virchow-Zentrum für Experimentelle Biomedizin der Universität Würzburg
22.06.2017