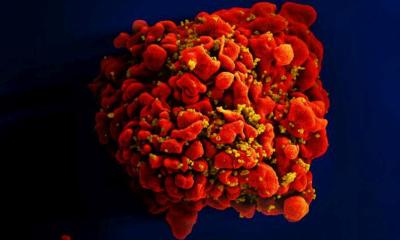
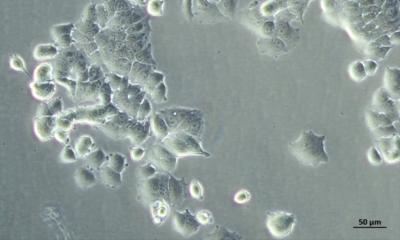
Heart Muscle Cells
Molecular strut controlls mechanics of a heartbeat
On top of the meaning and mystery that humans heap on the heart, it is first and foremost, a muscle. And one that beats about once a second for a person’s entire life, with no rest. Given its vital importance, it’s ironic researchers have only recently made direct observations of its subcellular parts in motion.

Now, using new high-resolution microscopy, a team from the Perelman School of Medicine and the School of Engineering and Applied Science at the University of Pennsylvania found that molecular struts called microtubules (MT) interact with the heart’s contractile machinery to provide mechanical resistance for the beating of the heart. Their findings, which could have implications for better understanding how microtubules affect the mechanics of the beating heart, and what happens when this goes awry, are published this week in Science.
Microtubules of the cell’s inner support system have diverse structural and signaling roles in heart muscle cells. Alterations in this microtubule network have been suggested to contribute to heart disease, but just how microtubules behave in the beating heart is poorly understood.
Direct observation of MTs during contraction is the most straightforward way to shed light on their contributions to heart function. The team observed temporal and spatial changes in MT shape during heart contraction. In addition, recent studies suggest that chemical changes to the MTs, called detyrosination (the removal of a tyrosine chemical group), regulate mechanotransduction, but it was unclear how.
Under Pressure
“We asked whether detyrosination alters how microtubules respond to changing physical pressure each time the heart contracts and relaxes,” said senior author Ben Prosser, PhD, an assistant professor of Physiology. “To answer these questions we used advanced imaging techniques to explore microtubule behavior in beating heart muscle cells from rodents.”
Under the microscope, cardiac muscle looks like interconnected bundles, termed myocytes. Heart myocytes are narrower and much shorter than skeletal muscle cells, with many mitochondria that produce the energy needed for all of that beating over a lifetime. “Heart muscle tissue is highly organized and that’s why it’s such an efficient machine,” Prosser explained.
During contraction the somewhat stiff MTs must somehow accommodate to the changing shape of myocytes. In this new study, the researchers found that in a typical myocyte this shape shift is accomplished by the MT deforming into a buckled spring that returns to an identical resting configuration after each beat. MTs are directly connected to the contractile machinery called sarcomeres. This link is at least partly accomplished via a protein called desmin that anchors MTs to the sarcomere, giving it a lattice-like structure.
The physical link between MTs and the sarcomere is highly dependent on detyrosination, so they tweaked the MT-sarcomere system to locate its weakest link. The team found that in myocytes in which detyrosination was suppressed MTs often accommodated contraction by sliding past each other rather than buckling as the sarcomere shortened. Disrupting the MT-sarcomere interaction allowed the sarcomere, and thus the heart cell, to shorten farther and faster, as well as decreased the cell’s overall stiffness.
“We speculate that there should be some optimum set point to the system and pushing it too far in either direction could be detrimental,” Prosser said. “The heart is a carefully honed machine that often operates within a narrow regime and pushing it outside this regime is often what leads to dysfunction over time.”
Conversely, they found that increasing detyrosination increased myocyte stiffness and impeded contraction. “This was a seeing-is-believing moment,” Prosser said. “We can now really observe how too many stiff microtubule struts would be detrimental to the heart over time. Our bioengineering coauthors precisely measured the microtubule contribution to cellular mechanics and quantified mathematically how this affected heart cell performance.”
"We developed a mathematical microstructural model to analyze how active contractile forces lead to buckling of microtubules and to quantitatively interpret the measurements from Prosser lab and validate their hypothesis,” said Vivek Shenoy, PhD, a professor of Materials Science and Engineering.
Clinical Relevance
The team’s findings are consistent with clinical data that shows a direct correlation between excess detyrosination and a decline in heart function in patients with hypertrophic cardiomyopathy (thickened heart muscle). The team found that detyrosination was greater in diseased hearts, by comparing human heart tissue donated from heart transplant patients with normal heart tissue from brain-dead donors, obtained from transplant cardiologist and coauthor Ken Margulies, MD, a professor of Medicine.
Cells from diseased hearts had more MTs, and these MTs showed more detyrosination. This process correlated with impaired function within this patient population in that their whole hearts, before the transplant, had a lower ejection fraction that correlated with greater detyrosination.
“Essentially we found that excess detyrosination promotes the interaction between microtubules and the sarcomere, increasing resistance to contraction and so may contribute to reductions in cardiac function in certain disease states.” Prosser said. “If we can break the microtubule crosslink, then the sarcomere can shorten more easily without the stiff, connected microtubules. We want the microtubules to slide back and forth more smoothly, which lets the sarcomere and therefore the heart to contract more, allowing the heart to pump blood more efficiently. This is useful to counteract the higher blood pressures outside the heart seen in many patients. Overall we want to increase the heart’s contractility and beating strength.”
The team is now asking whether specific drugs that reduce detyrosination can make diseased heart muscle beat stronger. They have tested this in mice and it works as predicted. Their next step is moving on to human diseased heart cells.
Source: The Perelman School of Medicine
28.04.2016