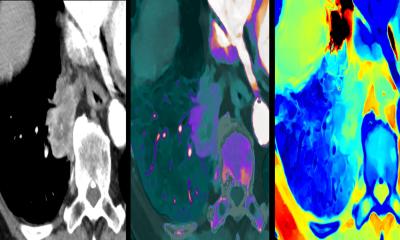
Microbubbles and ultrasound to treat ischemic stroke
A new research collaboration to study the potential of microbubbles and ultrasound to treat ischemic strokes has been established between ImaRx Therapeutics, a biopharmaceutal company in Tucson, Arizona, and the Philips' Medical Systems division in Andover, Massachusetts.
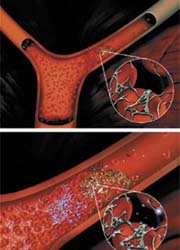
Philips ultrasound technology is to be used within the ImaRx’s SonoLysis programme* to develop new treatment for acute ischemic stroke. Under the agreement, Philips will provide ultrasound devices and technical assistance to ImaRx during its laboratory and preclinical studies using the ImaRx’s proprietary MRX-801 microbubble technology to determine the optimal ultrasound parameters to be used.
The vast majority of strokes, According to the American Stroke Association, about 87% of strokes are ischemic, i.e. caused by blood clots that block normal blood flow in brain vessels. Additionally, Datamonitor reports that less than 6% of the ischemic stroke patients receive the thrombolytic drug tPA, the only drug currently approved by the FDA to treat acute ischemic stroke.
‘This research collaboration represents a significant step forward for ImaRx’s SonoLysis programme,’ added Bradford A Zakes, President and CEO of ImaRx Therapeutics. ‘By working closely with Philips Medical Systems, we are incorporating the leading ultrasound technology and expertise into the early stages of product development, which strengthens our position as we move further through our clinical trials.’
Anne LeGrand, senior vice president and general manager of Ultrasound, for Philips Medical Systems, confirmed that Philips recognises the promise of microbubble therapies.
The companies’ agreement includes a mutual exclusivity clause during the term of the collaboration. Following completion of the research programme, Philips and ImaRx will have an exclusive negotiation period to discuss future development and commercialisation.
The ImaRx’ SonoLysis research programme
The company focuses on developing and commercialising therapies for stroke and other vascular disorders. (Current commercialisation efforts are focused on its product, Abbokinase as a treatment for acute massive pulmonary embolism).
The aim of this research programme is to develop product candidates that involve the use of the firm’s proprietary MRX-801 microbubbles with ultrasound to break up blood clots and restore blood flow to oxygen deprived tissues. The sub-micron size of MRX-801 microbubbles may allow them to penetrate a blood clot, so that when ultrasound is applied their expansion and contraction, or cavitation, can break the clot into very small particles, ImaRx reports. ‘ImaRx is conducting an ongoing Phase I/II multinational clinical trial evaluating its SonoLysis+tPA therapy to treat patients with acute ischemic stroke.’
14.11.2007