DNA
Keeping the cellular production line on track
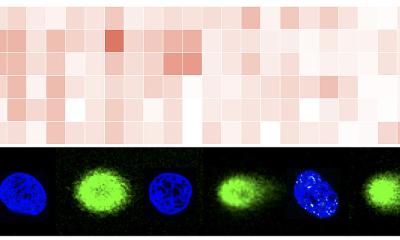
When our cells copy their DNA to grow and replicate, it’s vital the process runs smoothly. To get this right, cells use a complex “machine”, made from many hundreds of components. This machine is built afresh, moment by moment as it’s needed during the copying process. This assembly–de-assembly is vital to accurate and efficient copying.
Scientists thought they had a fairly clear understanding of how this happens. Now, a team at the MRC’s Clinical Sciences Centre at Imperial College, has found that one key protein has a different and far more important role than had been thought. When the team blocked the protein, called Cdc6, the machine jammed and DNA copying stopped – the cellular production line had broken down.
The findings, published today in eLife, may one day help to improve cancer treatments. Current chemotherapy drugs kill cancer cells by damaging their DNA, but they can also harm the DNA in healthy cells. This can cause mutations that ultimately drive the cells to develop into secondary tumours. If scientists could develop a treatment that targets the cellular machinery that copies DNA, instead of targeting the DNA itself, they may be able to reduce the risk of such side effects.
Blocking Cdc6 could be the answer, says Christian Speck who leads the DNA Replication group at the CSC, and who is one of the study’s senior authors. But first, scientists need a clearer understanding of exactly how DNA replication works.
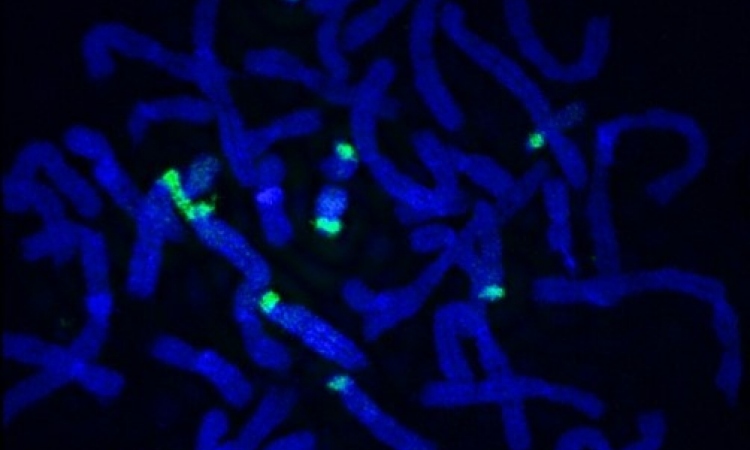
Our DNA is stored as two strands that are tightly twisted together and joined like a zip. The most important information sits in the middle of this twisted structure, or in the teeth of the zip, where it’s protected from harm. To copy this information, the strands need to be temporarily pulled apart. To do this, the cell recruits a large number of proteins and assembles them into a complex copying machine. A key component in the machine is a ring-shaped enzyme, called DNA helicase, which acts a bit like the clasp on a zip that allows you do it up or un-do it. The helicase unzips the DNA by binding to a strand and pulling it through the centre of its ring, but it is not yet known exactly how the helicase binds to the DNA strand.
It was thought that Cdc6 acts like a tiny motor to place the helicase on top of the strand. Speck and his collaborators have overturned this theory by showing that the helicase can still get into position even when the Cdc6 motor is turned off. However, without active Cdc6, the cell was unable to proceed to the next stage in the copying process – the machine was jammed. This showed Cdc6 is vital for the machine to function, but it was still not clear exactly what it does.
The study shows that, once the early stages of copying have begun, the Cdc6 motor is required for removal of the helicase assembly machine. When the researchers inactivated the Cdc6 motor, the machine remained in place for too long.
“Imagine that you leave a spanner in an engine, or don’t remove some of the tools that you used to assemble it. The engine will become jammed and stop working,” says Speck. “So Cdc6 motor activity makes sure there’s no spanner in the works, and keeps the production line going. It’s a sort of quality control protein.”
Regulated assembly and disassembly ensures that the machine copies the DNA only once. Speck says “If you replicate the DNA more than once, you end up with gene duplications – and this can cause cancer.”
Speck and his collaborators from the Van Aandel Research Institute in Grand Rapids, Stony Brook University in New York and the Brookhaven National Laboratory, also in New York, have developed a pioneering method to investigate DNA replication. First they studied proteins, including the helicase and Cdc6, which had been extracted from Saccharomyces cerevisiae (bakers yeast) and purified. Then they explored how these proteins behave in living yeast cells, to learn how they fit into the broader picture of cell activity.
The genes that encode these proteins are similar in yeast and people, so understanding the function of Cdc6 in yeast may help scientists to work out how to inhibit it in human cells – and ultimately block DNA replication.
The Speck lab is using high-resolution electron microscopes to examine the intricate details of a protein’s structure. The CSC team use 3D printers to create large-scale models of these protein complexes. These models help them to visualise how the tiny protein components come together to form the complex copying machine.
The next steps in the research will be to find out exactly how Cdc6 carries out its function, and to investigate how the same proteins behave in people. This will be the focus of future grant applications.
Source: MRC Clinical Sciences Centre/Institute of Clinical Sciences (ICS) Faculty of Medicine, Imperial College London
09.09.2015