Genome Structural Variation Consortium used Roche technology
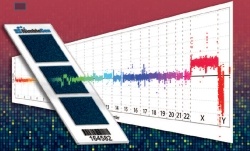
Roches NimbleGen´s CGH microarray platform was used to generate the highest-resolution map of human genome copy number variation. Recent advances in microarray technology have led to the discovery of extensive copy number variation in the human genome, including DNA copy number gains (duplications), losses (deletions), and multiallelic or complex rearrangements.
Copy number variants (CNVs) have been shown to contribute to inherited genetic disease and to confer resistance to infection, but the full extent of CNVs in the human population and the role of CNVs in common, complex diseases (e.g. diabetes, cardiovascular disease, psychiatric disorders) is not yet well understood.
The Genome Structural Variation Consortium, which includes researchers from the Wellcome Trust Sanger Institute (Hinxton, UK), Brigham and Women’s Hospital & Harvard Medical School (Boston, MA, USA), and the Hospital for Sick Children (Toronto, Canada), used a set of twenty NimbleGen HD2 arrays, each array containing 2.1 million probes for a total of 42 million probes, to extend the current human genome CNV map down to 500 bp resolution, a ~100-fold improvement over the map released in 2006 (Redon et al. Nature 444:444, 2006). Data from this study, which included analysis of 20 females of European ancestry (CEU HapMap samples) and 20 females of African ancestry (YRI HapMap samples), has recently been made available as a public resource (http://www.sanger.ac.uk/humgen/cnv/42mio/).
According to Gerd Maass, CEO of Roche NimbleGen, “We’re honored that NimbleGen 2.1M arrays were used to generate the data for this essential public resource. Although the scientific research community has yet to fully understand the entire contribution of CNVs to common, complex diseases, we have seen that many genetic diseases within families often result from CNVs, and the presence of other CNVs may protect individuals with other diseases, including HIV and malaria. We believe that these data and other genomic information that help to map mutations will provide insight into how CNVs contribute to disease susceptibility and resistance in an individual, a group, or a population.”
28.01.2009