Article • Kickstarted imaging
First total body PET/CT scanner cleared for clinical use
The first total-body positron emission tomography/computed tomography (PET/CT) that can acquire a 3D image of the human body in a single position received 510(k) clearance from the U.S. Food and Drug Administration (FDA) in January 2019.
Report: Cynthia E. keen
Its forthcoming commercial availability for clinical use in the United States later this year is the milestone achievement of a multi-institutional consortium led by two medical physicists at the University of California-Davis and its commercial collaborator, United Imaging Healthcare, headquartered in Shanghai, China. Total-body PET scanners are expected to revolutionize and significant expand clinical utilization of the technology. Not only will they acquire a whole-body image, but they will produce images of higher quality, with less radiation dose, and in a much faster time period.
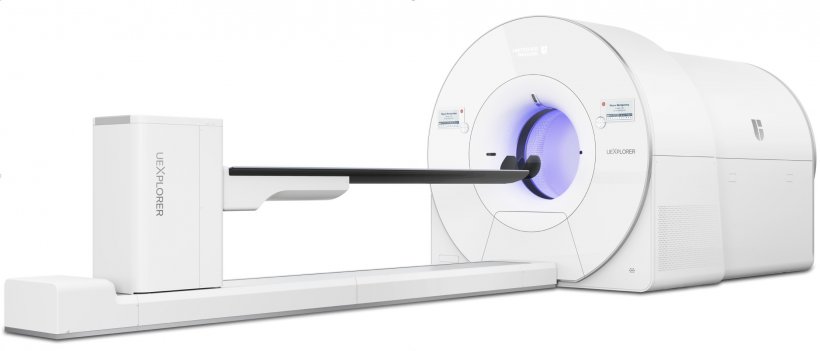
The uEXPLORER PET/CT scanner can produce a whole-body diagnostic scan in as little as 20 to 30 seconds compared to 10-20 minutes with a conventional PET scanner. It can scan up to 40 times faster than a conventional PET scanner, or use up to 40 times less radiation dose. A 40-fold increase in dynamic range enables the imaging of radiotracers by more than five half-lives, enabling repeat imaging of a patient without the need for additional injection of radiotracers.
The UC Davis research team, jointly led by Professor Dr. Simon R. Cherry and Ramsey D. Badawi, began in 2005. A $15.5 million grant from the U.S. National Institutes of Health in October 2015 which included funding from the National Cancer Institute (NCI) and the National Institute of Biomedical Imaging and Bioengineering (NIBIB) kickstarted the EXPLORER project. Ultimately, several prototypes were designed and tested by engineers and researchers from UC Davis, the University of Pennsylvania in Philadelphia, and the Lawrence Berkeley National Laboratory in Berkeley, California.
Two smaller scale “mini-EXPLORER” PET scanners were developed for biomedical research and companion animal studies. The contract to fabricate the total-body scanner for human use was awarded to United Imaging Healthcare America of Houston, Texas, in January 2017. This scanner was completed in May 2018. The first human images, acquired in collaboration with the Department of Nuclear Medicine at Zhongshan Hospital in Shanghai, were shown in a scientific session of the 2018 RSNA annual meeting. In parallel, a team at the University of Pennsylvania has also developed a prototype human scanner with a 70 cm axial field of view with extremely high time-of-flight performance.
The total-body PET allows maximal detection of the radiation from injected radiotracers emitted from the body. By increasing the geometric coverage to include the entire body, the sensitivity of the scanner can be increased by a factor of about 40 for total body imaging or a factor of about 4-5 for imaging a single organ. Dr. Cherry said that this sensitivity can be used to increase the signal-to-noise ratio in reconstructed images, supporting reconstructing images at higher spatial resolution and potentially allowing the detection of smaller or lower contrast lesions. The increased sensitivity gain can also be used to reduce imaging times by a factor of up to 40 or to reduce the injected radiotracer by a factor of 40. This latter attribute could potentially make the use of PET more feasible for infants and children, or allow up to 40 scans of a patient for the same effective dose as currently received in a single scan. A 40-fold increase in sensitivity means that a tracer could be followed for 5-6 additional half-lives, enabling additional or longer scanning from a single injection.
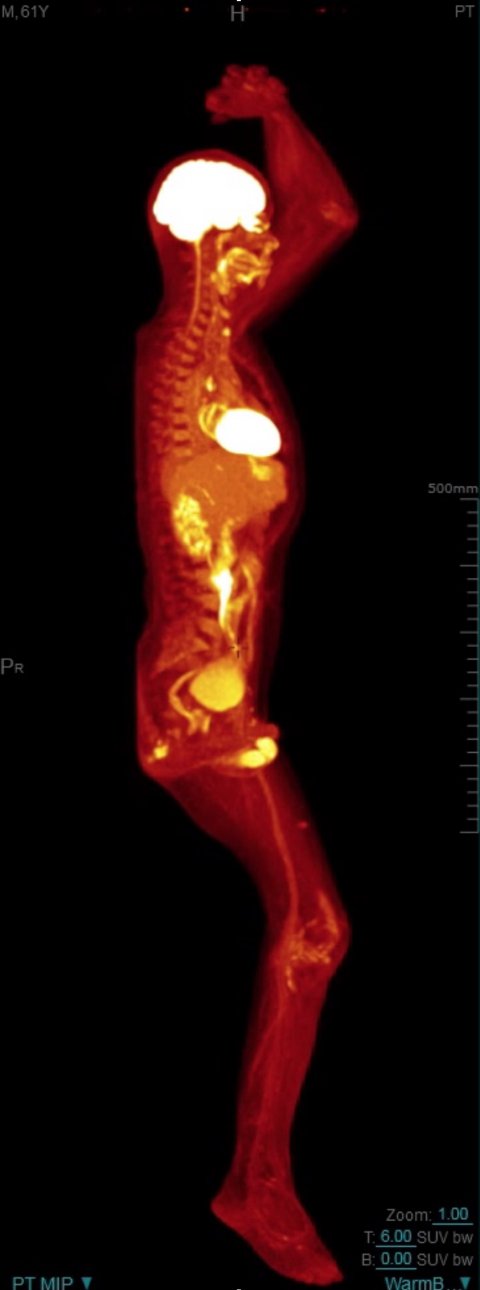
Dr. Badawi showed the high level of detail of the clinical images of the uEXPLORER in a scientific session at RSNA 2018. He suggested that the total-body PET/CT scanner may be used for imaging of total-body tumor perfusion and peripheral vascular disease. It may be able to identify small cancer deposits in the body, and also be used for immunotherapy planning and dosimetry. The potential of detection of lower numbers of cells and particles could enable tracking of the effectiveness of cell-based therapies and provide information on the delivery and retention of nanoparticles in nanoparticle-mediated therapies.
Benefits of a total-body PET scanner for children is that low-dose scanning would be feasible, and ultra-fast scanning could reduce or eliminate the need for anesthesia. Scanning would be improved for morbidly obese patients. Extremely low radiation dose scans may even make fetal imaging feasible.
In an article published in the Journal of Nuclear Medicine, the development team wrote that “the primary driving motivation for developing total-body PET was originally clinical research”. They suggested that the greater expense of the system compared to a conventional PET scanner could be offset by the fact that there could be greater utilization within a radiology department, with up to 40 patients in 15-minute time slots imaged daily. Additionally, the cost of radiotracers may decrease and be more cost-effective if lower injected quantities can yield high quality images.
The first uEXPLORER will be installed at UC Davis, and clinical use is anticipated in mid-2019.
26.02.2019