Image source: HKUMed
News • Accelerated bone-to-implant integration
Responsive coating could fast-track implant integration after surgery
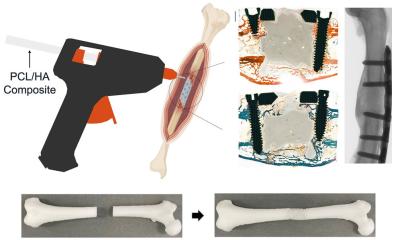
A research team led by Professor Kelvin Yeung Wai-kwok from the Department of Orthopaedics and Traumatology, School of Clinical Medicine, LKS Faculty of Medicine, the University of Hong Kong (HKUMed), has developed an innovative photocurrent-responsive implant surface to accelerate bone-to-implant integration after orthopaedic surgery.
The cutting-edge coating has been shown to shorten the integration time to just two weeks, doubling the speed and expediting post-operative recovery as well as reducing the risk of rejection. The team is currently exploring the application of this technology in artificial joint replacement surgeries, including commonly performed knee replacement surgeries in Hong Kong. The findings have been published in Advanced Functional Materials.
Disorder in the osteoimmune microenvironment during the post-implantation phase can result in implant loosening, extending recovery time and increasing postoperative complications, ultimately resulting in implant failure. To address these challenges, the HKUMed team has developed a near-infrared (NIR) light-responsive implant surface that positively influences the macrophage response, effectively reducing acute inflammation during the crucial early post-implantation stage. This process involves the generation of a photocurrent in response to NIR light, which instigates increased calcium influx in macrophages, creating a more favourable osteoimmune microenvironment. This enhances the recruitment of mesenchymal stem cells (MSCs) and promotes bone formation, thereby speeding up the bone-to-implant integration process.
Animal experiments have proved that this method significantly accelerates bone-to-implant integration, resulting in a twofold increase in the fusion rate
Kelvin Yeung Wai-kwok
When an implant is introduced into the body, it triggers a complex response, known as foreign body reaction (FBR). This intricate process involves various cellular and molecular events that determine the effectiveness of bone-to-implant integration. Macrophages, which play a pivotal role in the FBR, are among the first immune cells to respond, initiating a chain reaction that is crucial for bone-to-implant integration. Upon insertion, these immune cells become activated and trigger an acute inflammatory response, releasing pro-inflammatory cytokines, like tumour necrosis factor-alpha, to facilitate the recruitment of mesenchymal stem cells (MSCs) and initiate the bone-regeneration process.
This inflammation process begins immediately upon implantation and peaks within a day or two. However, in a case in which the host immune system's self-regulation is compromised due to a local pathological condition, the spike in inflammation may not be adjusted in time and may progress to chronic inflammation. This could result in a range of complications, including the formation of fibrous capsules, bone resorption, enzymatic degradation of implants, or a delay in integration, eventually leading to implant failure. Alarmingly, over 10% of implant failures are linked to implant loosening. Therefore, it is critical to restore a balanced environment between the bone and the implant, particularly after the initial inflammation phase, to prevent long-term inflammation and ensure successful implant integration.
Orthopaedic implants are typically coated with titanium dioxide (TiO2), which is non-toxic to bone cells and bacteria, but has limitations in its responsiveness to NIR. Known for its ability to penetrate biological tissues, NIR is widely used in antibacterial and cancer treatments. In this study, the research team used hydroxyapatite (HA), the primary component of bone and teeth, to develop an excitable surface that responds to a photocurrent. This novel coating generates photoelectric signals when exposed to NIR, which swiftly reduce acute inflammation and actively regulate macrophage differentiation, creating a beneficial immune environment tailored to the patient’s condition to foster bone-to-implant integration. This regulation promotes the recruitment of mesenchymal stem cells for bone formation, ultimately accelerating bone-to-implant integration, making the implants more secure.

Image source: HKUMed
In experiments using a tibial defect animal model, the team found that bone-to-implant integration was accelerated from 28 days to just 14 days, effectively doubling the speed. This marks the first study that uses a photocurrent to non-invasively regulate immune cells. This discovery is expected to advance the development of new biomaterials capable of remotely controlling the local immune environment.
‘Our team has successfully developed an engineered surface that non-invasively modulates macrophage differentiation according to the patient's immune cycle and needs,’ said Professor Kelvin Yeung Wai-kwok, who led the research. ‘Animal experiments have proved that this method significantly accelerates bone-to-implant integration, resulting in a twofold increase in the fusion rate. We aim to expand the application of this engineered surface in orthopaedic surgeries in future research to enhance patient recovery. This discovery has a profound impact on the success rate of orthopaedic surgery and provides a new direction for addressing clinical challenges, like implant rejection.’
Source: University of Hong Kong
18.12.2024