News • Glioblastoma
Building a better model for brain tumours
A research team led by the University of Alabama at Birmingham is launching a study to find a better model for glioblastoma, a particularly devastating type of brain tumour, to help determine the most appropriate treatment modality.
Credit: Christopher Willey, M.D., Ph.D.
The $3.6 million, five-year U01 grant award is funded by the National Cancer Institute, one of the National Institutes of Health. The UAB team will join four other institutions with similar U01 awards — University of California-San Francisco, Duke University, University of Utah, and Cold Spring Harbor Labs/Jackson Labs, to form the Patient-derived Models Consortium, or PDMC.
Neurospheres and microtumours allow for high-throughput screening of potential drugs, which should produce a cheaper and quicker way to identify compounds that might have therapeutic benefit
Christopher D. Willey
Use of the NCI-60 has been phased out in favor of patient-derived xenografts, or PDX, in which a patient’s own tumour cells are grown in a mouse model. Xenograft models permit cancer cell interactions with other cells and conditions that are not currently fully replicated in a laboratory dish. Creating and testing patient-derived xenografts is a slow and expensive process, so the NCI is looking for the most effective ways to use the xenografts and related models to identify new ways to fight cancer.
The UAB-led research team is examining two new technologies, neurospheres and microtumours, and will compare them with PDX. “Neurospheres are self-assembling groups of cells that can be grown in defined media conditions,” said Christopher D. Willey, M.D., Ph.D., associate professor, Department of Radiation Oncology in the School of Medicine. “Microtumours involve embedding the same type of tumor cells into a matrix material in a three-dimensional structure. Both allow for high-throughput screening of potential drugs, which should produce a cheaper and quicker way to identify compounds that might have therapeutic benefit.”
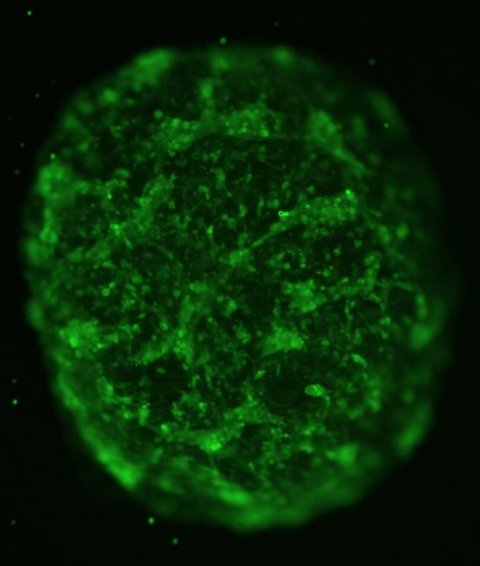
The study will attempt to better understand how the various models — neurospheres, microtumors or PDX animal models — are influenced by their growth conditions in terms of their molecular signaling and sensitivity to cancer treatments. “Our intent is to see how these systems respond to typical therapies, such as chemotherapy or radiation,” said Yancey Gillespie, Ph.D., professor emeritus in the Department of Neurosurgery. “We will be able to directly determine which genes are being expressed and which enzymes are active in the same tumour cells growing in three different environments. This data will allow us to change the models or their environments to mimic the brain tumour environment in the patient and then assess how each model responds to therapy. The idea is to make a more accurate, patient-like model or models for human brain tumours.”
“Some treatments we thought would work well in brain tumour patients have not been as effective as we’d hoped. We believe that we can identify better therapies if we have a model that most closely represents actual tumours in a human brain,” said Anita Hjelmeland, Ph.D., associate professor in the Department of Cell, Developmental and Integrative Biology. “Using the improved model, we can better understand which drugs will work best for a particular patient with a particular type of cancer.”
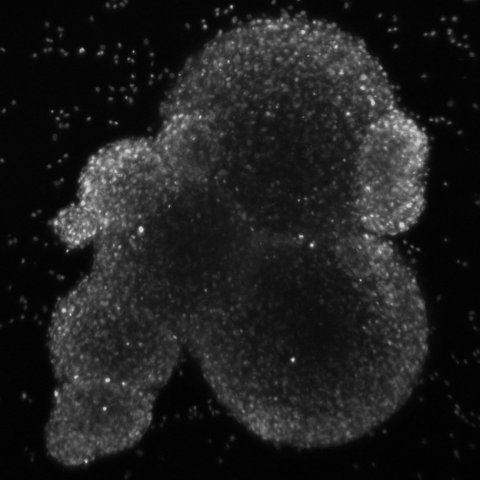
The team acknowledges that there may not be one “best” model. “To characterize these PDX models, we plan to apply an array of new computational tools,” said Jake Chen, Ph.D., professor in the departments of Genetics, Computer Science and Biomedical Engineering and chief bioinformatics officer of the UAB Informatics Institute. “Genomic, transcriptomic and kinomic data will be systematically collected from these glioblastoma PDX models. We will develop new bioinformatics and systems biology techniques to integrate all these quantitatively measured signals — hundreds of thousands of them for each model — to build ‘in silico’ models. These models can then be manipulated in computers rapidly and help us predict each tumor’s subtype and drug sensitivity behaviors before actual experiments are performed.”
The study’s findings could help to guide better therapies in the future for brain cancers such as glioblastoma, the kind of tumor that affected John McCain and Ted Kennedy. Glioblastoma is the most common and most aggressive primary malignant brain tumor, with an expected survival of less than 15 months.
Source: University of Alabama at Birmingham
22.10.2018