Source: Loyola Medicine
News • landmark study
Bladder cancer: Robotic surgery is as effective as open surgery
Robotic surgery is as effective as traditional open surgery in treating bladder cancer, according to a news study. 350 patients participated in the nationwide trial, who were randomly assigned to undergo robotic surgery or open surgery to remove cancerous bladders.
After two years, there was no significant difference between the two groups in survival without disease progression. Robotic surgery was associated with less blood loss and shorter hospital stays, but longer surgeries. There were no significant differences in complication rates or in patients' quality of life. The study is called RAZOR (randomized open versus robotic cystectomy trial) and was funded by the National Cancer Institute.
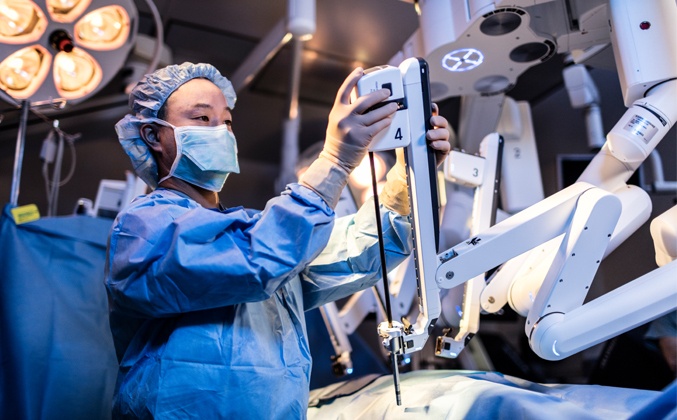
A robotic system allows a surgeon to perform operations through a few small incisions. Movements by the surgeon's hand or wrist are translated into highly precise movements of the surgical instruments. Every maneuver is directed by the surgeon, in real time, as the surgeon views a magnified, 3D, high-definition image of the surgical site.
Since robotic surgery was introduced in 2000, it has spread rapidly and has been used in about four million surgeries worldwide. But apart from the RAZOR trial, there have been no prospective, randomized multicenter trials to assess how robotic surgery compares to open surgery in cancer survival.
The study is titled "Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an open-label, randomized, phase 3, non-inferiority trial."
The RAZOR trial found that two years after surgery, 72.3 percent of patients in the robotic surgery group were alive, with no disease progression and essentially cured, compared with 71.6 percent in the open surgery group. Sixty-seven percent of robotic surgery patients experienced adverse effects such as urinary tract infections and intestinal obstructions, compared with 69 percent in the open surgery group.
Robotic surgery patients stayed a median of six days in the hospital, compared with seven days in the open surgery group. Robotic surgery patients lost less than half as much blood as open surgery patients, but spent more time in the operating room (seven hours, eight minutes compared with six hours, one minute).
Researchers wrote that the findings "underscore the need for further high-quality trials to assess surgical innovation before this surgical technique is widely adopted in clinical practice."
Dr. Gupta, co-author of the study, added that the study provides evidence demonstrating that the robotic approach performs at least as well as the open approach. "It is important to conduct these trials before widespread adoption of technology, as has been the case with robotic prostatectomy (removal of the prostate)," he said.
Source: Loyola Medicine
16.07.2018