News • Laboratory operations
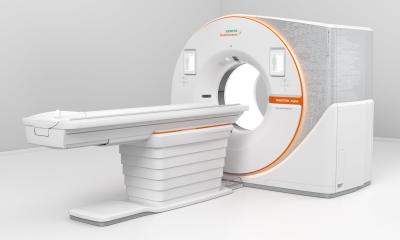
Atellica NEPH 630 System now available
Siemens Healthineers announced its Atellica NEPH 630 System* is now available to laboratories. The Atellica NEPH 630 System is a low- to mid-volume nephelometric protein testing solution that simplifies laboratory operations by unifying instrument, assay, IT connectivity and remote service disciplines to deliver advancements in protein testing.
Nephelometry is a laboratory technique used to quantify specific levels of proteins found in blood and other bodily fluids. Information on the concentration of these proteins is important in assessing and monitoring cardiovascular risk, kidney diseases, neurological disorders, nutritional status and other diseases, such as multiple myeloma. While many systems only support protein level assessment on plasma or serum, the Atellica NEPH 630 System offers a broad menu of protein tests for assessment of urine, cerebrospinal fluid, plasma and serum, enabling enhanced quantification of certain health conditions for advanced clinical decisions.

“The Atellica NEPH 630 System combines advanced technologies and innovative assays to address the continuous pressure that labs are under to streamline workflow, improve cost efficiency, and drive better outcomes for clinicians and patients,” said Franz Walt, President, Laboratory Diagnostics, Siemens Healthineers.
Proven nephelometric technology provides high precision, reproducibility and pre-reaction protocols for high antigen-excess security. These are essential components for result accuracy and minimize the need for repeat testing. Because advanced antigen-excess checks, single-use cuvettes and kinetic curve displays are incorporated into the instrument, labs can report results with confidence. To accommodate different testing volumes, the Atellica NEPH 630 System offers multiple reagent package sizes, resulting in less waste for laboratories. Additionally, lot-to-lot reagent consistency ensures concordance between results, providing physicians with greater insight into the patients’ disease progression.
* Not available in the U.S. Product availability varies by country.
Source: Siemens Healthineers
17.10.2017