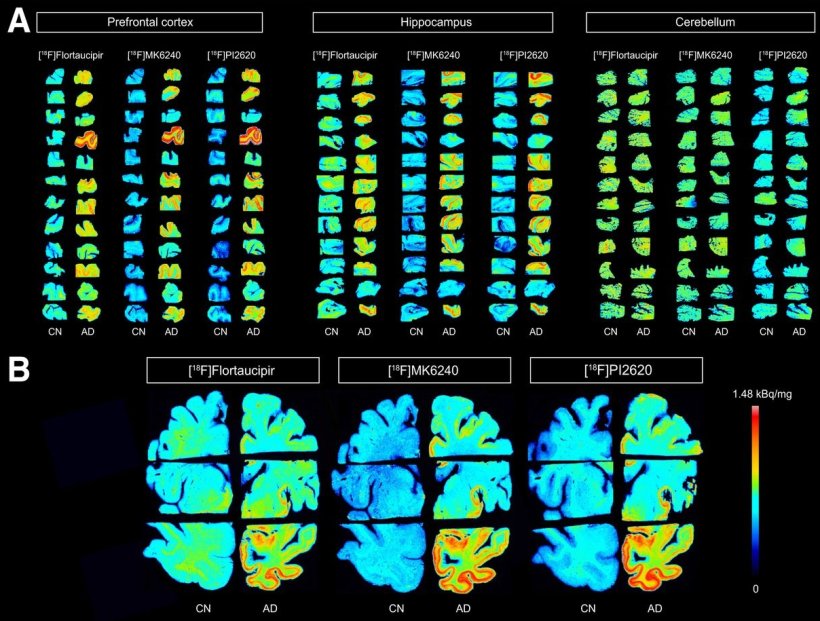
Autoradiography images showing binding of [18F]flortaucipir, [18F]MK6240, and [18F]PI2620 in prefrontal cortex, hippocampus, and cerebellum (A) and in whole-brain hemisphere (B) of control and AD brains. CN = control.
Image source: SNMMI; from: Aliaga A, Therriault J, Quispialaya KM et al., Journal of Nuclear Medicine 2025
News • Promising imaging agents
Alzheimer's disease: New radiotracers for better detection
Two new PET radiotracers have outperformed the only currently Food and Drug Administration (FDA) approved radiotracer for detecting tau tangles in the brain, a hallmark of Alzheimer’s disease.
In a head-to-head comparison of the three imaging agents, the next-generation radiotracers exhibited higher binding to Alzheimer’s disease brain tissue and a greater selectivity for identifying the tangles. This research, which was published in the Journal of Nuclear Medicine, could play a key role in measuring outcomes of clinical trials for Alzheimer’s disease treatments.
In Alzheimer’s disease, tau tangles are correlated with cognitive impairment: the more tangles, the more severe the impairment. By quantifying the amount of tau in the brain tissue with PET imaging, physicians can appropriately stage Alzheimer’s disease in patients and determine the best treatment options. “Identifying tau tangles is crucial for diagnosing and staging Alzheimer’s disease,” said Pedro Rosa-Neto, MD, PhD, director of the Translational Neuroimaging Laboratory in the Douglas Research Centre at McGill University in Montreal, Quebec. “In our study we tested three different tau radiotracers to determine how well they could discriminate late-stage Alzheimer’s disease brain tissue from healthy brain tissue.”
With their higher specificity, these new tau imaging agents are ideal for detecting the small changes that occur in brain tissue over time
Eduardo R. Zimmer
In the study, autoradiography was used to assess the binding of 18F-Flortaucipir (FDA-approved), 18F-MK6240 and 18F-PI2620 in autopsy-confirmed Alzheimer’s disease and healthy brain tissues. Binding values were calculated based on regions of interest in the prefrontal cortex, hippocampus, and cerebellar cortex sections of the brain, as well as the whole-brain hemisphere.
For all three radiotracers, a significant difference in binding was noted between the Alzheimer’s disease brain tissue and the healthy brain tissue in the whole brain hemisphere, prefrontal cortex, and hippocampus, but not the cerebellar cortex. Binding to Alzheimer’s disease brain tissue was higher for 18F-MK6240 and 18F-PI2620 than for 18F-Flortaucipir. 18F-MK6240 and 18F-PI2620 also had greater selectivity than 18F-Flortaucipir. “With their higher specificity, these new tau imaging agents are ideal for detecting the small changes that occur in brain tissue over time,” noted Eduardo R. Zimmer, PhD, assistant professor of pharmacology at the Universidade Federal do Rio Grande do Sul, in Porto Alegre, Brazil. “This can be especially helpful in Alzheimer’s disease clinical trials that utilize tau PET as an outcome measure.”
Additionally, the study indicates that harmonization methods are needed to circumvent the differences between tau imaging agents. “Our work represents an important step toward the harmonization of tau tracers,” Zimmer said, “and the results might provide insights into initiatives to create a universal scale for tau tracers.”
Source: Society of Nuclear Medicine and Molecular Imaging
31.01.2025