Aging and death
by Andeas Simm
A medical failure or biological necessity?
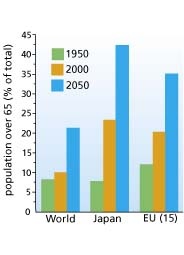
Europe will see a drastic increase in its aged populations by 2055, and with this an increase the percentage of age-related illnesses will become an even greater burden on healthcare. Whilst researchers seek cures, we also need to understand the biology of aging, says Dr Andreas Simm, biologist and lecturer affiliated with the Martin Luther University Halle-Wittenberg medical faculty and the German Society for Thoracic and Cardiovascular Surgery’s Working Group for The Elderly Patient in Heart Surgery.
“In a 100 years average life expectancy has more than doubled globally (men: from 25-65 years; women up to 70 years), mostly due to better hygiene, nutrition and infection cures. In the first half of the 1900s infant mortality fell dramatically and, in the second half, life expectancy also improved, particularly for the elderly.
However, over 100 years ago the oldest person, Thomas Peters, reached 111-year-old (1745-1857). More recently Jeanne Calment became the oldest authenticated person dieing aged 122-year-old (1875-1997). So, despite the augmentation of years for an average person, the maximum age reached has changed little. Researchers worldwide estimate the number of supercentenarians, i.e. over 110 years, to be under a thousand (www.grg.org/Adams/E.HTM).
We can conclude that our maximum age appears to be genetically determined. Depending on how and where we live, maximum extended life will remain between 110 and 120 years, but actual life expectations will be environmentally determined - or, better, by changes in the way we live.
Aging can be defined as the loss of the capacity of homoeostasis, i.e. an increasing incapacity to adapt to a fast-changing condition. For example, the young can handle unexpected and sudden physical exertions easily, whereas older people have difficulty. This seems valid for the psychical load capacity as well. Many old people report reduced mental mobility. This does not mean they are incapable of high performance. They simply need more time. The population often places aging as a synonym for age-associated diseases, particularly cardiovascular diseases (at about 50%, the frequent cause of death in the western industrial nations), tumours (at about 25%, the second most frequent cause of death) and Alzheimer’s, a well-known example of degenerative diseases of the brain.
What causes those diseases? It is important to put some trust in biology. To ensure humans continue as a species, Nature ensures we reproduce when young, healthy and strong. S J Olshansky (School of Public Health, University of Illinois) compared the human body with a racing car, constructed to reach its aim as quickly as possible, without needing repairs. Although a certain reserve is always built in, what finally counts is only the distance between the beginning and end. By contrast, a human should reach reproductive age (aim), then live on, aging only from his reserve. This analogy is not absolutely wrong; we see that very old animals are not usually found in the wild, but are in a protected environment, e.g. the zoo. Similarly, for a long time the average life expectancy of humans was around 25 years. There are indications that the same mechanism and/or gene functions that provide the biggest advantages in the period from birth to reproduction also actively contribute in great age to common aging diseases. This is described as pleiotropic antagonism - one of the evolution-based theories of aging.
This will serve as an example of the replicative senescence in relation to the origin of cancer. Tumours originate mainly from mitotic cells (self dividing). Long-living organisms with renewable tissues have many such cells and so a higher risk of tumorigenic transformation. This transformation increases exponentially with age. Most of the age-dependent tumours are carcinomas and originate from epithelial cells. Tumour cells, or premalignant cells (precursor of tumour cells), have requirements in order to grow as a tumour, which include an accumulation of damage to cell molecules, such as the lipids, proteins and particularly DNA. DNA mutations accumulate with age and heaped up, to be found as tumours. However, it was shown that such genomic changes/instabilities often appear already in benign tumours or early tumour stages. This means that for the growth of a tumour, additional components are important - and one is the micro-environment, where potential tumorigenic cells are found. Stroma cells, e.g. particularly cells of connective tissue (fibroblasts), are responsible for the micro-environment of tumour cells. For example, if one injects embryonal stem cells into adult tissues, they could develop teratocarcinoma. On the other hand, these cells are capable of reproducing normal tissues after injection into an embryo.
According to the environment in which tumour cells are found, their growth can be blocked - or promoted. Components for this environment - extracellular matrix proteins - are synthesized by fibroblasts. During aging, these synthesize another spectrum of proteins and contribute to the changing environment. Senescent fibroblasts enrich themselves in tissue with age and are demonstrable e.g. through the expression of an age-associated acid galactosidase activity. At Berkeley, the Campisi team showed that the growth of premalignant epithelial cells could be strongly stimulated by co-culture of senescent and not young fibroblasts. If premalignant breast epithelial cells are injected with fibroblasts in an immune deficient mouse, big lethal tumours originate only from co-injection with senescent fibroblasts.
Fibroblast aging, known since the 1960s, was first described by Leonard Hayflick (San Francisco) as replicative senescence: in a culture human cells divide themselves up to 80 times, then lose the capacity for cell division. A possible cause of this is the shortening of telomeres (ends of the chromosome) with each cell division. However, cellular senescence is also a programme that is induced through potential tumorigenic stimuli, such as radiation, radical and heterochromatic disturbances. This has been described as operating as a tumour suppressor mechanism. The induction of cellular senescence is disturbed in transgenic mice, which often die early from tumours. Consequently, this is a classical example for a pleiotropic antagonism. Cellular senescence avoids tumours in the early years, however, such aged cells enrich themselves during tissue aging and can promote the growth of tumours through a changing micro-environment.
Georg Wick, at the Institute for Biomedical Aging Research, Innsbruck, describes other substantial examples of this. These include the reaction of inflammation due to arteriosclerosis. The human immune system is extremely effective in fighting infections in youth, when antibodies against the bacterical heat-shock-protein HSP60 are formed. If the endothelium (internal blood vessel layer) is damaged by necrosis, for example due to high blood pressure, these antibodies react against human HSP and can cause a chronically auto-immune response. In the lung, RAGE, the receptor of the Advanced Glycation Endproducts (AGE) appears to function as a tumour suppressor. However, chronic activation in old age contributes to an inflammatory response and diseases such as arteriosclerosis.
The concept of antagonistic pleiotropism may give some explanations as to how a beneficial mechanism that effectively protects the body in youth, contrarily transforms itself in aging. That is why aging, on the cellular level, cannot be renounced - it is a biological necessity and not a failure of a medical disposition. If we disturb or switch off these mechanisms, humans would probably die before reaching the reproductive age. Biological mechanisms point to how we could treat age-related diseases more effectively in the future. Despite improved disease treatments, human aging, per se, actually cannot be influenced. Nowadays, the only proven and effective possibility to prolong life is caloric restriction. If animals are deprived of up to 50% of their calories, about 30% of life prolongation has been observed. All other possibilities of ‘ anti-aging medicines’ should be views with caution, because they either do not work or, as in hormone therapy, have side effects.
Given the circumstances, we could conclude that aging is an aspect of our private lives. Finally, aging is also important for us as a species, because it affect evolution, which works through the renewal of generations. Otherwise we would still live the sad existence of early cavemen.
07.08.2006