Image source: Texas A&M College of Engineering
News • Antithrombotic approach
A new way to detect blood clots
Biomedical engineering researchers at Texas A&M University designed a medical device that mimics blood vessels to design and monitor drugs for patients with clotting disorders.
This approach could be especially beneficial for pediatric patients. The researchers published their findings in the journal Scientific Reports.
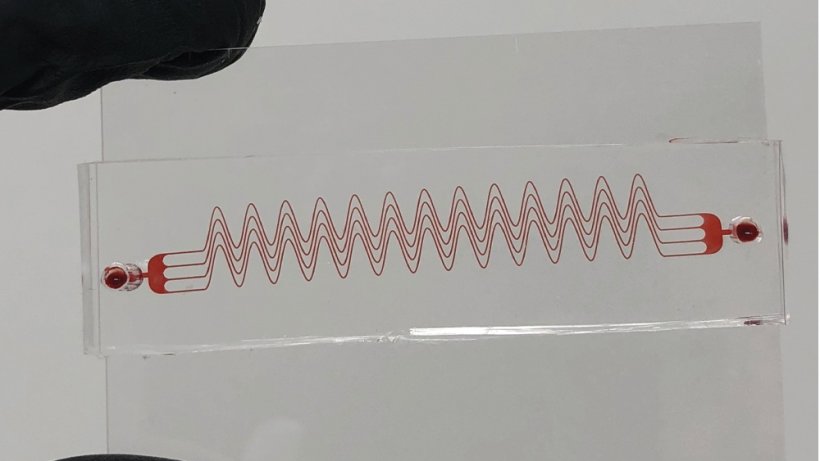
Unlike what a biology textbook may show, blood vessels are not straight cylinders. They are tortuous, meaning they have complex curves, spirals and bends. When the blood reaches these curves, it makes changes to its fluid mechanics and interactions with the vessel wall. In a healthy person, these changes are in harmony with the tortuous microenvironment, but when diseased, these environments could lead to very complex flow conditions that activate proteins and cells that eventually lead to blood clots.
Assistant Professor Abhishek Jain said a large challenge in medicine is that the medical devices used to detect clots and assess anti-blood clotting drug effects are entirely chemistry-based. “They do not incorporate the flow through the naturally turning and twisting blood vessels, which are physical regulators of blood clotting,” Jain said. “Therefore, the readouts from these current static systems are not highly predictive, and often result in false positives or false negatives.” To approach the problem from a new angle, researchers in Jain’s lab at Texas A&M designed a microdevice that mimics tortuous blood vessels and created a diseased microenvironment in which blood may rapidly clot under flow. They showed this biomimetic blood clotting device could be used to design and monitor drugs that are given to patients who suffer from clotting disorders.
Jain said he can see several applications for the device, including critical care units and military trauma care units. “It can be used in detection of clotting disorders and used in precision medicine where you would want to monitor pro-thrombotic or anti-thrombotic therapies and optimize the therapeutic approach,” Jain said.
The margin for error is essentially zero for these [anticoagulated] patients
Abhishek Jain
After developing the device, the team took it into the field for a pilot study. Working with Dr. Jun Teruya, chief of transfusion medicine at Texas Children’s Hospital and Baylor College of Medicine, the team coordinated with clinicians to test the device with pediatric patients in critical care whose heart and lungs were not working properly. These patients were in need of an extracorporeal membrane oxygenation (ECMO) machine, which provides cardiac and respiratory support in exchange of oxygen and carbon dioxide. A common complication in ECMO is blood clotting, so patients are administered anticoagulants to prevent clotting. However, ECMO machines are also known to “eat” clotting proteins and platelets, which puts anticoagulated patients in further risk of bleeding. Anticoagulated pediatric patients on ECMO are especially prone to bleeding.
Current chemically based blood clotting tests are expensive, time-consuming, can be unreliable and require a skilled technician. Jain’s team’s tortuosity based microfluidic system doesn’t require expensive chemicals, is quick, with results within 10-15 minutes, uses low blood sample volume and is easy to operate. “The margin for error is essentially zero for these patients,” Jain said. “Therefore, it’s imperative that all the tests, not just clotting tests, must work and provide clinicians with quick and reliable information about their patient so they can provide the best care possible.”
By having the opportunity to test their system with real patients, Jain said his team was able to demonstrate that their design could detect bleeding in anticoagulated patients with low platelet counts, which can help guide doctors to make better evidence-based clinical decisions for their patients. For Jain and his team, the next stage is continued clinical studies to compare their approach to standard methods and hopefully demonstrate key performance advantages.
Source: Texas A&M University
06.06.2020