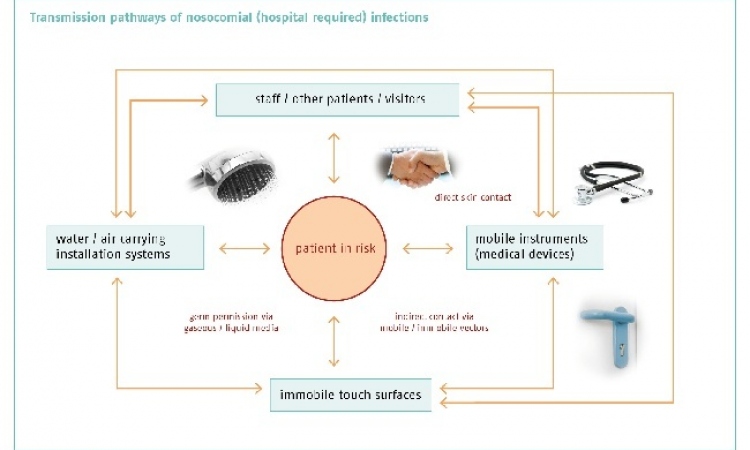
Nosocomial infections
Can we win the struggle against HAIs?
According to ECD statistics for Europe, three million cases of nosocomial infections occur annually, and 50,000 are fatal. Evelina Tacconelli MD PhD (below) is Assistant Professor of Infectious Diseases at the Università Cattolica Sacro Cuore in Rome, Italy. Her scientific focus is on epidemiology, clinical and therapeutic aspects of nosocomial infections and infection control policies aimed to reduce the spread of infections caused by antibiotic resistant bacteria, and her work has been awarded for research excellence by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Dr Tacconelli is a member of the ESCMID Executive Committee and the Editorial Board of its publication Clinical Microbiology and Infection. She also serves as an expert for the National Committee for the prevention of catheter-related infections, and is a member of the Cochrane Wound Group. Speaking with European Hospital, Dr Tacconelli assessed the current level of control over potentially lethal pathogens and continuing efforts to combat the problem.

Thus far efforts to control the spread of Methicillin-resistant Staphylococcus aureus (MRSA) have failed worldwide, with a few exceptions (the Netherlands), which has resulted in a great deal of public and political concern. Of even more concern are recent reports of S. aureus isolates recovered from chronic haemodialysis patients that are resistant to vancomycin and linezolid.
To develop effective prevention strategies we must understand the various components required to achieve the endemic state. To make it simpler, the endemicity of hospital-acquired infections (HAIs) due to MRSA are caused by a constant influx of bacteria into the healthcare setting from newly admitted patients who are colonised or infected with MRSA, followed by cross-transmission between hospitalised patients with de novo acquisition and efflux of MRSA from the hospitals into the community after patients’ discharge. Up to now, the majority of prevention strategies have focused on the middle component, as with hospital guidelines for antibiotic therapy and prevention of cross-transmission between hospitalised patients and staff.
In recent years, MRSA is being discovered with increasing frequency at hospital admission. These ‘community-acquired’ MRSA strains arise from two different patient populations: those with ‘true’ community-acquired MRSA strains, which have emerged de novo from community-based S. aureus strains in specific populations, such as children, inmates, and military personnel, and ‘healthcare-associated’ strains, which have been acquired in a hospital during a recent exposure to the healthcare setting or surgical procedures.
This changing epidemiology has led many hospitals in Europe and the USA to introduce universal screening to detect MRSA colonisation in patients at hospital admissions, in order to reduce the influx of MRSA into the hospital and therefore HAIs. For several years conventional culture methods with enrichment cultures, which can take at least 48-72 hours to obtain a result, have been considered the gold standard to detect MRSA colonisation. However, in the absence of pre-emptive room isolation, that time might be enough to spread MRSA among hospitalised patients. Recently, rapid methods for molecular detection of MRSA colonised patients with polymerase chain reaction (PCR) have been developed with available results in two hours. However, studies on the impact of these new tests, which are more costly than conventional cultures (around £10 versus £2 per single test) showed conflicting results.
Ten studies have been published (nine interventional studies and one randomised trial). Four studies reported a significant decrease in the rate of MRSA infections while four observed no effects. Two studies reported a significant decrease of MRSA infections in medical wards, but not in surgical wards. The only randomised clinical trial observed no change in MRSA infection, while the only study using universal screening (the other 9 applied screening only in selected wards) reported a significant reduction of MRSA disease. We have just completed a systematic review and meta-analysis that includes all the above mentioned studies. Results, to be published on Lancet Infectious Diseases, further suggest that the available evidence on the efficacy of these new tests for MRSA screening at hospital admission is still poor and definitive recommendations cannot be made.
A number of questions remain unsolved for the treatment of severe MRSA infections (bacteraemia, endocarditis, osteomyelitis, pneumonia). Although the ‘old’ drugs (vancomycin and teicoplanin) still represent the drugs of choice, there are several concerns on the treatment of MRSA infections: reports of clinical failure with vancomycin treatment regardless of in vitro susceptibility; increasing reports of strains with reduced vancomycin susceptibility; difficulty in therapeutic dosage monitoring; lack of evidence on the efficacy of combination therapy. Moreover, in recent years pharmaceutical companies have curtailed the pipeline for the antibiotics production. Linezolid, daptomycin and tigecycline are the only three really innovative drugs to treat MRSA infections produced in the last 20 years. Linezolid might look like the more versatile of the drugs because it also can be given orally. Although clear indications exist only for its use in skin and skin-structure infections and pneumonia, linezolid might be considered a valid alternative treatment for MRSA bacteraemia. The drug’s potential to cause bone marrow suppression limits its use, especially for a duration longer than two weeks. Among the new developed antimicrobials, Daptomycin is the only drug approved for MRSA bacteraemia and endocarditis. Tigecycline, the latest developed drug, looks promising. However, its role in the treatment of hospital-acquired pneumonia is still unclear. At the moment, the availability of these new drugs, although they have definitively improved options for MRSA treatment, should be carefully monitored, to avoid future development of resistance (already detected).
In Italy, a national surveillance, performed in 2004 by National Institute of Health (ISS) in 50 hospitals, showed that almost 500,000 patients (5–7% of all in-patients) develop HAIs and 15,000 die annually. Over 50% of these infections are caused by antibiotic-resistant bacteria, including MRSA. Although specific data are not available, in my experience the majority of Italy’s university hospitals follow international guidelines for MRSA (including isolation in flagged or single rooms when possible; cohorting in case of large clusters; promotion of hand hygiene; use of dedicated material in gowns, gloves, masks, and the safe disposal of all potentially contaminated material). A survey developed by the Multidisciplinary Italian Society for the Prevention of Infections in Healthcare Organisations(SIMPIOS) showed that, in more than half of the interviewed hospitals, targeted MRSA screening at hospital admission were in use in selected wards (e.g. cardiothoracic and orthopaedic surgery) or ICUs.
In an interesting new project (just started in my hospital, a 1,700-bed university hospital, Policlinico Agostino Gemelli, in Rome), a dedicated group of ID specialists, experts on nosocomial infections, has been created. Every day, physicians receive e-mails from the micro laboratory giving alerts to target antibiotic-resistant micro-organisms (including MRSA) isolated all over the hospital. The infected patient is then visited by the expert who decides on the kind of infection control measure needed, and therapy and follow up are provided by a dedicated infection control (IC) nurse.
The first goal of the task force is to differentiate between ‘true’ infection and ‘colonisation’ (not requiring therapy). This may be particularly difficult in high-risk populations: the severely immuno-compromised and ICU patients. We expect that such a programme, that also includes education designed on the specific ecology of the wards, will lead to a reduction in inappropriate therapy, cross-transmission, and eventually reduction of HAIs, including those caused by MRSA.
01.07.2009