Eucomed statement on PIP breast implant incidents
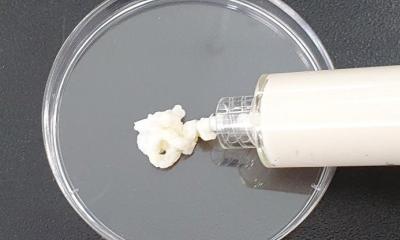
The European medical technology industry association Eucomed notes with great concern recent press reports that the French firm Poly Implant Prothèse (PIP) may have violated the regulations by using unapproved industrial-grade silicone in some of its implantable products and by allegedly falsifying or withholding documents.

If these or other allegations are confirmed, Eucomed strongly condemns PIP’s behaviour and breach of trust. Patient safety, public confidence, and ethical behaviour should always be the top priorities of any organisation active in the healthcare industry.
Launched in 1990, the current European regulatory system for medical devices has proven to ensure a high level of safety and quality of medical devices, while making innovative medical technology available faster than elsewhere in the world. Unfortunately, however, no system can entirely guard against fraud or deliberate abuse. Eucomed supports effective measures that prevent a similar case happening in the future. The European regulations for medical devices are currently under revision and Eucomed has submitted proposals that would strengthen and modernise the current system (please see the appendix for more details).
Breast implants for medical purposes are regulated in Europe as ‘Class III’ medical devices. As such, they attract the strictest pre-market controls by independent assessment bodies. Those bodies (called Notified Bodies) are appointed and overseen according to pan-European requirements by national authorities, which are also responsible for post-marketing surveillance. The controls include pre-market review of the manufacturer's design and safety data for the product (called a design dossier), which includes material safety and compatibility and clinical evaluation based on clinical data.
The manufacturer’s facilities, records, and quality management system are also subject to regular audits by the responsible Notified Body, typically every 6-18 months. The manufacturer is legally obliged to ensure that any changes to the approved design, specifications, materials, or manufacturing processes are supported by documented evidence to prove continued compliance with the safety and performance requirements. Planned changes are reported to the Notified Body for further review.
Whilst continuing to support the main elements of the current regulatory model, which have proven to be highly effective, Eucomed recognises the need to modernise and strengthen the current medical devices legislation in Europe. It believes this should be done by coupling enhanced Member State engagement with better science-based coordination and management of the regulatory system at European level. The proposed changes should lead to a smart and efficient legislative framework that is consistently implemented across the EU and guarantees high quality healthcare, patient safety and rapid access to the latest value-based medical technologies.
Eucomed is on record as calling for a legal framework that provides a consistent, EU-wide regulatory approach through improved coordination, evaluation and certification of medical devices; consistent and comprehensive implementation across all EU Member States, as well as efficient vigilance and market surveillance systems. Europe needs smart regulation that makes efficient and effective use of existing resources and involved structures (European Commission, Competent Authorities, Notified Bodies and industry) so as to retain a simple, adaptable and highly efficient system and merit public confidence.
Six steps to a smarter legal framework for medical devices:
1. Only the best Notified Bodies;
2. One approach to vigilance and market surveillance;
3. Strengthened harmonised standards;
4. Consistent implementation of guidelines;
5. Increased transparency;
6. An integrated approach: better coordination and management.
09.01.2012