Article • Microbleed detection
A new hope for Alzheimer's prediction
New information on dementia biomarkers is emerging, as increasing results from population studies become available. However, although the list of risk factors lengthens, the value of these predictors, and more generally the cause of disease, remain to be determined, according to Gabriel Krestin, professor and chairman of the Department of Radiology & Nuclear Medicine at Erasmus MC, University Medical Centre Rotterdam.
Report: Mélisande Rouger

Are brain microbleeds predictors of dementia? Ever since radiologists spotted microscopic hemosiderin deposits on MRI, their association with the development of Alzheimer’s disease (AD) is increasingly acknowledged. Gabriel Krestin is the architect of population imaging in the Rotterdam Study, a large epidemiological cohort of 15,000 elderly subjects who have been followed for more than 25 years. He coordinated the installation of a dedicated imaging infrastructure 12 years ago and, since then, more than 14,000 brain MRI and 3,000 CT scans have been carried out to find out what brain features are associated with the development of dementia. For Krestin, there is no doubt that microbleeds and dementia correlate.
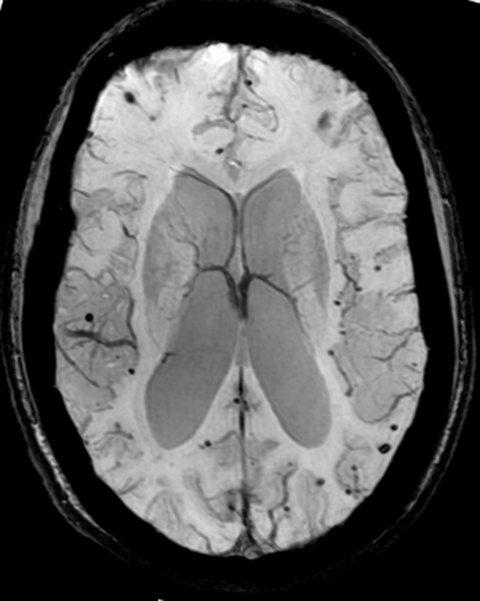
There is also a strong association between incidence of microbleeds and the use of antithrombotic drugs. But the prognostic value of these haemorrhages has yet to be clearly demonstrated to issue proper recommendations, he explained. ‘What is the predictive value of microbleeds? Should we recommend all our patients not taking aspirin? A lot of attention has been given to this information since over the past years. Truth is, we still don’t know for sure.’
There is a consensus on several risk factors and associated predictive imaging biomarkers of AD, and the best known is hippocampus atrophy. ‘There is a relation between the size of the hippocampus and the development of neurodegenerative disease. The smaller the hippocampus is, the earlier on the onset of dementia. The risk of developing dementia increases in individuals with smaller volumes of the hippocampus and amygdala,’ Krestin explained. Volume changes of the hippocampus are even stronger predictors of dementia, and Krestin and his team have shown that accelerated atrophy is even a stronger predictor of AD. ‘Even the shape of the hippocampus and the fact that not all its parts are decreasing at the same pace may predict AD development,’ he added.
‘There is an association between microbleeds, or signal voids, and cerebrovascular disease and the development of dementia. Here we have a potential predictor of an increased risk and development of disease,’ he said. Thanks to MR techniques using susceptibility imaging, researchers involved in the Rotterdam trial have detected a significant portion of microbleeds – enough to be able to prove the link between these lesions and not only AD, but also overall mortality. ‘Even one single microbleed increases the risk of mortality; if there are more than five, mortality significantly increases, and so do cardiovascular and other diseases. Microbleeds are a strong predictor of increased mortality,’ Krestin confirmed.
Analysing white matter microstructure
The integrity of white matter microstructure is a very strong predictor
Gabriel Krestin
White matter lesions (WML) and silent lacunar brain infarcts are additional acknowledged biomarkers. Early on, the Rotterdam Study showed that the number of lesions was related to cognitive decline. ‘The more WML there are, the faster the cognitive decline of the subjects is. Increased periventricular WLM load is associated with cognitive decline and the risk of dementia,’ he said ‘There is also a relationship between silent or small brain infarcts and AD.’
Even when radiologists don’t see anything, they can measure alterations of the white matter microstructure thanks to diffusion-weighted imaging (DWI). According to Krestin, such ‘underwater’ or invisible markers are associated with impaired brain executive functions and may also predict the development of dementia. His team is now focusing on assessing the microstructure of the white matter to detect invisible lesions in that area. ‘The integrity of white matter microstructure is a very strong predictor. Even before lesions are visible, diffusion parameters are measurable and predict the development of disease,’ he explained.
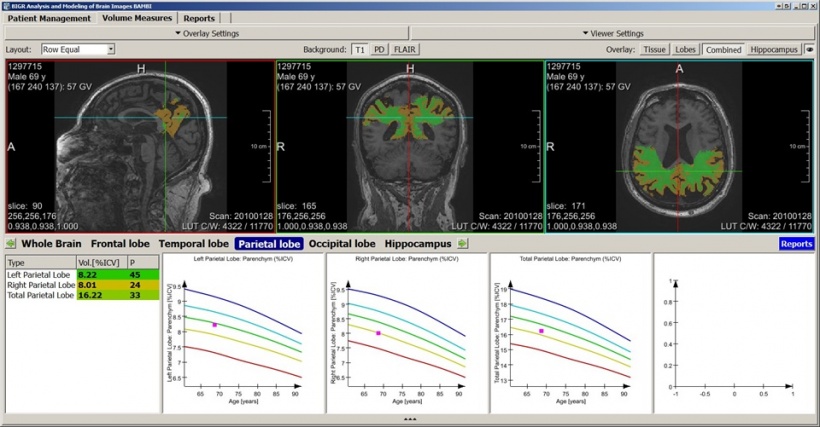
The integrity of white matter microstructure is a very strong predictor
Gabriel Krestin
Using probabilistic tractography, the Dutch researchers have shown that lower tract microstructure integrity was associated with cognitive decline. Krestin believes they could even go a step further and establish relations between the brain’s functions and its microstructure and other functions of the body, since there are some associations between kidney function and probably cardiac function and brain microstructure. The professor and his colleagues are now looking into the disconnectivity hypothesis, i.e. integrating structural MRI and resting state fMRI to build up to the so-called connectome. ‘We want to look at these connections and the function of white matter tracts in order to predict development of cognitive decline at an earlier stage.’
Meeting the challenges of harmonisation
Genome-wide association studies (GWAS) may contribute to finding associations between genetic alterations and particular imaging biomarkers. However, there are many challenges ahead. ‘You need extremely large populations and to pull the data from different cohort studies possibly including 20-50,000 subjects, to find significant genotypic alterations related to certain imaging phenotypes. You also need a high level of harmonisation in terms of image acquisition, sequencing and data processing,’ he added.
The Rotterdam Study collaborates in the CHARGE consortium, including large population cohorts in the Netherlands, Iceland and the USA. A network of researchers in epidemiology genetics and imaging that cooperates to better understand neurodegenerative brain disease. For instance, they found a mutation on chromosome 17, which is associated with WML. They are not sure how to interpret this information yet. ‘Is this genetically determined? Is the presence of such mutations associated with earlier dementia onset? We don’t know,’ Krestin said. GWAS are only showing associations with and increased risks of developing certain diseases, but not necessarily the cause of disease, he pointed out. ‘The utility of these studies is unclear. We find out something about pathophysiology, but not really the cause of this association yet.’
Profile:
Professor Gabriel P Krestin MD PhD is professor and chairman of the Department of Radiology & Nuclear Medicine at Erasmus MC, University Medical Centre Rotterdam, The Netherlands. He is also the Scientific Director of the European Institute of Biomedical Imaging Research (EIBIR).
27.02.2018