Trauma care
Using high energy ultrasound to control internal bleeding
By Shahram Vaezy, Associate Professor of Bioengineering at the University of Washington, Washington, Seattle, and Vesna Zderic, Assistant Professor at Electronic and Computer Engineering Department at George Washington University, Washington DC.
In the ongoing quest for technologies that result in as little damage as possible when used in surgery, therapeutic ultrasound stands out because it can offer what no other technology does: non-invasive, bloodless ablative surgery. Whether it is an emergency operation to staunch deep internal bleeding, or an elective procedure to remove a tumour, ultrasound has been shown to provide an effective method that can deliver ablative energy to deep-seated tissues, while requiring no incision in the skin or surgical exposure of the tissues of interest.
This technology, dubbed High Intensity Focused Ultrasound (HIFU), relies on the ability to focus ultrasound waves on a region about as small as a grain of rice, positioned at any desired depth in soft tissues. The main difference between HIFU and diagnostic ultrasound imaging is in the levels of applied acoustic intensity, while both use similar frequencies in the range of 1-10 MHz. In ultrasound imaging, low intensity waves of about 0.1 W/cm2 are used to reflect from tissue structures to be resolved in the image, and in HIFU, high intensity waves of about 1000 W/cm2 are used to cause instant coagulative necrosis and tissue disruption.
The application of HIFU to the haemorrhage control problem (often called acoustic haemostasis) stems from the need to have an effective method to stop bleeding in ‘the golden hour’ after severe traumatic injury, involving profuse bleeding. Many trauma patients suffering injuries with high bleeding rates expire during transportation to hospital, or on the operating table, due to haemorrhagic shock (particularly true for battlefield victims).
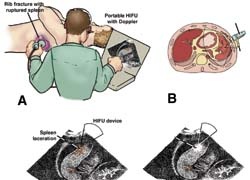
In general, cessation of haemorrhage using extrinsic, interventional methods is possible with delivery of energy to bleeding tissues, i.e. cauterisation. We have shown in preclinical studies in large animals that bleeding from injuries of liver, spleen, kidneys, and major blood vessels (femoral and carotid arteries, jugular vein, aorta, etc) can be stopped using HIFU within a minute or so. The mechanisms of acoustic haemostasis include thermal and potentially mechanical effects. Temperatures above 70 degrees Celsius can be produced in the targeted tissue within seconds. Further, it appears that boiling of interstitial fluids and blood, as well as acoustic cavitation (i.e. formation of microbubbles at the focus due to mechanical HIFU effects) are also involved in acoustic haemostasis. The biological effects are believed to include coagulative necrosis due to high temperatures, and mechanical disruption of tissue structures potentially leading to release of tissue factors enhancing the coagulation, coagulum and thrombus formation at a wound site, tissue fusion via collagen and elastin remodelling, and fibrin plug formation.
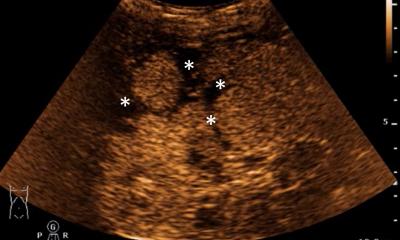
HIFU integrated with ultrasound imaging offers a unique potential for developing a small portable device that can be brought to the site of an accident. Such a device can be used to detect and localise the bleeding site using ultrasound imaging and to stop the bleeding using HIFU, all in real-time, using a seamless image-guided therapy system. Currently, the ultrasound imaging systems can be as small as a laptop and the HIFU devices can fit in a small suitcase.
Further development of these devices would provide automation of the bleeding detection and treatment methods to facilitate its usage by paramedics and other first responders. A haemorrhage control device for use outside hospital settings shortly after injury occurs, and capable of site-specific treatment, could provide a life- and limb-saving tool in trauma management.
Contacts: adasi@u.washington.edu
zderic@gwu.edu
14.11.2007