Stem Cells
Current research and the future of regenerative medicine
By Professor Gustav Steinhoff MD, director of the Department for Cardiac Surgery, and Christof Stamm MD, co-ordinator of clinical studies, at Rostock University, Germany
Cell therapy for myocardial regeneration is an exciting new field of medical research that has the potential to revolutionise cardiovascular medicine. Despite significant improvements in emergency treatment, myocardial infarction leads to a loss of contractile tissue in many patients with coronary artery disease. Often, this is the beginning of a downward spiral towards heart failure and life-threatening arrhythmia. Other than heart transplantation with its obvious limitations, current therapeutic means aim at preventing further episodes of myocardial ischaemia and at enabling the organism to survive with a heart that is working at a fraction of its original capacity. They are far from representing a cure. At present, the fast emerging research field of stem cell technology opens a new vision of regenerative therapies for heart disease. In this situation, it is understandable that cardiac stem cell therapy attracts considerable attention and has raised many hopes.
Stem cells are unspecialised cells that renew themselves for long periods of time and can be induced to become cells with a specialised function. The traditional definition requires the capacity for ‘asymmetric’ cell division (i.e. the stem cell divides into one stem cell and one differentiated cell), while a classic progenitor cell divides in two differentiated daughter cells. Whereas embryonic stem cells are uncommitted and pluripotent in their differentiation capability, adult stem cells are believed to be committed to differentiate only into specialised cells of the organ or tissue they are derived from. Understanding of adult stem cell biology has been upset by recent experimental data indicating that adult stem cells derived from the bone marrow can give rise not only to blood cells but also to other (non-haematopoietic) cell types by crossing lineage boundaries (‘stem cell plasticity’).
Ideally, a stem cell that has been implanted into infarcted myocardium would give rise to new blood vessels and new contractile cells (i.e. angiogenesis and myogenesis). In 2001, two experimental studies of myocardial repair by adult stem cells from haematopoietic sources, after experimental myocardial infarction, promoted an unparalleled boost of clinical and experimental regenerative SC therapy studies. Kocher et al. found that systemic intravenous infusion of purified human CD34+ cells can improve heart function in rats by generating new blood vessels within the infarct area (‘neo-vasculogenesis’), and our own group has recently reproduced this phenomenon using human cord blood cells. Orlic et al. reported on both neo-vasculogenesis and trans-differentiation of transplanted cells into cardiomyocytes following intramyocardial application of mouse bone marrow-derived stem cells. The initial enthusiasm, however, has largely faded. While in situ neo-angiogenesis induction by haematopoietic cells, associated with functional improvements, is consistently observed, it proved difficult to find corroborating evidence for true cardiomyocyte differentiation. In fact, two independent groups reported early in 2004 that they did not detect any meaningful evidence of cardiomyocyte differentiation of haematopoietic stem cells in mouse models that were designed to confirm the earlier findings.
Clinical application: Whether the first clinical pilot trials were initiated too early remains subject to very controversial debate. The first clinical application was reported by Menasche and colleagues. During an aorto-coronary bypass operation, skeletal muscle progenitor cells (‘skeletal myoblasts’ obtained from the patient’s own thigh muscle two weeks before the operation) were injected into the infarcted myocardium. This procedure has been repeated worldwide ever since, and the reports quite uniformly describe a mild improvement in heart function. The myoblast injection, however, appears to result in a transient period of electrical instability a few days after the injection, which repeatedly led to sustained ventricular arrhythmia. Therefore, myoblast injection as a stand-alone treatment is currently limited to patients who have an automatic defibrillation device implanted.
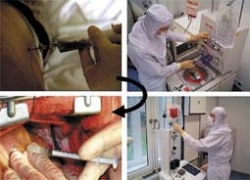
Cell therapy with bone marrow or blood-derived stem cells may evolve in a novel treatment option for both chronic ischaemic heart disease and acute myocardial infarction. In either situation, the angiogenic potential of certain adult stem/progenitor cell types is probably the key to functional improvements, whereas true neo-myogenesis is, at present, rather unlikely. Around 2001, several clinical trials were initiated, and among the first published was the work by Hamano et al, who injected bone marrow mononuclear cells intramyocardially during a CABG operation, Tse et al, who used a catheter-based system for direct intramyocardial delivery of mononuclear cells, and our own group, who at first injected a purified population of CD133+ bone marrow stem cells, again in conjunction with a CABG operation (Figure 1). What these trials have in common is that patients with chronic ischaemic heart disease are addressed. In the chronically ischaemic heart, still vital cardiomyocytes are dispersed within the fibrous scar tissue. Theoretically, such ‘hibernating’ cardiomyocytes can be re-recruited for contractile work once sufficient blood supply with oxygen and nutrients has been re-established, and stem cell-induced growth of microvessels in the infarct border zone may thus translate into improved myocardial contractility.
Over 40 patients in Rostock have been treated with bone marrow stem cells in the chronic ischaemic heart, which has proved safe and effective in improvement of cardiac perfusion and function, especially in those with previously deteriorated heart function.
It was fundamentally different in acute myocardial infarction studies. The onset of myocardial ischaemia was usually between several hours and a few days ago. If possible, the blocked coronary artery is immediately reopened by emergency catheterisation, balloon dilation, and stent placement. Analogous to the chronically hibernating myocardium, acutely ischaemic cardiomyocytes are still vital, but have temporarily lost much of their capacity for contractile work (‘myocardial stunning’). Tissue infiltration with inflammatory cells is beginning, but fibrous scarring has not yet occurred. In this situation, direct injection of cell suspension into the weakened myocardium is prohibitive, but infusion of stem/progenitor cells into the reopened coronary artery is currently being evaluated.
So far, mainly bone marrow mononuclear cell preparations have been used in such trials. A few days after the onset of myocardial infarction, a second cardiac catheterisation is performed and the cell suspension is injected into the infarct vessel while blood is temporarily interrupted by balloon inflation. Pilot studies have demonstrated feasibility and safety of this approach, and controlled efficacy trials are on the way. In one of the first of those trails, there was a difference in LV ejection fraction of 6% at six months follow-up between 30 patients who received intracoronary cell injection and 30 patients who only had standard infarct treatment. Other clinical trials based on the same principle are currently undertaken, but it is too early to make a definitive judgement about long-term functional efficacy and possible side effects. One of the most interesting questions is how, and to what extent, the intra-coronary cell injection leads to stem cell migration into the myocardial interstitium.
Outlook
Based on existing clinical experience, it should be justified to conclude that transplantation of autologous bone marrow cells in the heart can be safely performed in patients with ischaemic heart disease and leads to a functional recovery with improved cardiac perfusion. Whether neo-angiogenesis, neomyogenesis - or both - occur in the human situation as yet remains unclear. Carefully designed controlled studies are needed to further determine the efficacy of clinical cell therapy for heart disease. Scientific controversy regarding the regenerative potential of adult bone marrow stem cells in the heart of mice has fuelled a heated debate about further experimental and clinical approaches. However, given the tremendous amount of data demonstrating functional benefits in large animal models, the therapeutic potential needs investigation in further clinical trials.
In medical research, it is not unusual that novel treatment concepts are evaluated in a close combination of laboratory investigation and explorative clinical studies. Of course, every clinical trial must be designed and conducted with greatest care, to minimise patient risk and exclude ethical conflicts. If the translation of experimental approaches in clinical medicine succeeds, we will, for the first time, be able to offer patients with heart failure a true cure. The development of clinical stem cell therapy for different diseases, use and application of new knowledge from embryonic and adult stem cell research and the development of new pharmacological tools will guide the way to future applications of a regenerative medicine.
07.08.2006