Article • Non-invasive corona breath testing
SpiroNose: The electronic nose that knows about Covid-19
Rapid tests, PCR tests, self-tests… there are many test options to determine contamination with Covid-19. In most this is done by inserting a cotton swab deep into the nose and/or throat to extract some mucus – unpleasant for adults and often a drama for children. Towards the end of 2020, a new system emerged to rule out a Covid-19 contamination. The electronic SpiroNose performs a non-invasive breath test within a minute, with immediate result, the manufacturer reports.

SpiroNose research first began 15 years ago, at the LUMC and later the Amsterdam UMC. In 2018 several researchers there set up the company Breathomix to bring the SpiroNose from a research setting to clinical practice to diagnose lung diseases such as asthma, COPD and lung cancer. In 2019, research by Mirthe Muller (AVL), Rianne de Vries (Amsterdam UMC / COO of Breathomix) also showed that the SpiroNose can predict, with 85 percent certainty, whether immunotherapy will work in lung cancer patients.
This sensitive ‘nose’ attracted the attention of the Dutch Rijks Instituut voor Volksgezondheid en Milieu (RIVM) which approached Breathomix to see whether the breath test could be used to rule out corona infections. The Covid-19 test is therefore the first diagnostic application of the SpiroNose ‘Using the SpiroNose for this new application doesn’t require any adjustment to the hardware,’ Rianne de Vries pointed out. We do need to train the system for Covid-19. This is done by collecting many breath profiles of people with and without a Covid-19 infection to establish the reference standard and define the diagnostic model.’
To do so, in April 2020 Breathomix began a collaboration with the Leiden University Medical Center and Franciscus Gasthuis & Vlietland. The results of the first phase of that study, in hospitals, were very positive. On the advice of the Ministry of Health, Welfare and Sport (VWS) and in collaboration with the hospitals and GGD Amsterdam, several studies were started in two Covid-19 test streets from July 2020 onwards. Ultimately, about 4,500 people were tested for the validation of the SpiroNose, all of whom also received a PCR test as a control.
The fact that people with a negative result of the breathalyser no longer have to undergo a PCR test is more pleasant for them and it reduces the pressure on the testing lines
Rianne de Vries
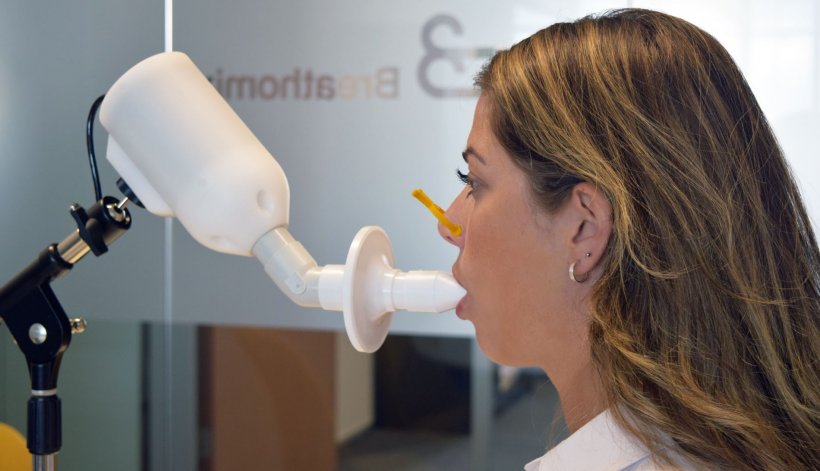
The electronic nose contains seven different sensors that can measure the complete mixture of substances (biomarkers) in the exhaled air. The test requires five normal breaths, followed by a deep inhalation, holding the breath for a moment and then exhaling slowly. A breath profile is created from that exhaled air and via a gateway sent to an online analysis platform. Within a few seconds, with the help of analysis algorithms, a test result will follow, indicating that the person tested is not infected with the virus. The SpiroNose therefore does not detect whether someone is infected but indicates with certainty that someone is not. About 70 percent of people get that negative result. The approximately 30 percent whom the device indicates cannot be assessed, or are not definitely negative, subsequently undergo a PCR or a LAMP test.
Having a dynamic start, the GGD stopped after a few weeks due to several unclear or false negative test results. ‘It was painful for us that the news incorrectly spoke of an unreliable test,’ said De Vries. ‘After reviewing the small number of unclear test results, it turned out that the problem was not technology-related but, despite the instructions, concerned human errors in the interpretation and registration of the test results. With new technologies it often takes some getting used to and learning how to deal with them. Together with various parties, we are looking at how to make the instructions even clearer. Fortunately, testing with the SpiroNose has resumed and the news has been put right.’
Recommended article

Article • Breath analysis to aid diagnoses
Breathomics: far more than hot air
In diagnostics, it sometimes makes sense to follow your nose. During the Labmed Forum at Medica, Dr Beniam Ghebremedhin and Dr Simona Cristescu discussed the diagnostic potential of breathomics – the analysis of a patient’s exhaled air for disease indicators.
The test gives immediate results and, unlike a PCR test, is non-invasive and much simpler. The speed allows the GGD’s to perform large numbers of tests. The costs are about two euros per test, excluding the efforts of the people who perform the test. Attention – People who drink alcohol: there must be at least eight hours between the last glass and the test. ‘The fact that people with a negative result of the breathalyser no longer have to undergo a PCR test is more pleasant for them and it reduces the pressure on the testing lines,’ De Vries pointed out.
Future opportunities
‘By continuously carrying out a PCR test on a small proportion of the people who tested negative,’ Rianne de Vries pointed out, ‘we keep a good view of the performance of the breathalyser in changing circumstances. This quality monitoring with the PCR test is important, which is, after all, the gold standard.’
The SpiroNose possibly can be used for children in the future. ‘Studies of SpiroNose have now been carried out in adults. With the Ministry of Health, Welfare and Sport, the LUMC and the Franciscus Hospital, we are investigating how best to do this, because, during the study, a comparison with a less pleasant PCR test must also be made and the question is whether parents are willing to take their children to it.’
Pilots are already running to use rapid tests at events and in companies. This will be expanded rapidly. In the future, possibly the SpiroNose could be used at airports, for example. The issue of test certificates is also being discussed. ‘Deployment at large-scale events does pose a logistical challenge that needs careful consideration. The equipment is expensive and must be used correctly – and time is a factor. If you are very skilled, you can have someone take the test, assess, disinfect, and replace mouthpieces and filters for new use in a minute or two. But, apart from the equipment, there are many other things to think about with the organisers. What do you do, for example, if someone has to undergo a PCR test afterwards? Facilities are also needed for that.’
The use of the SpiroNose at GGD testing facilities will be expanded in the short term: The Ministry of Health has purchased hundreds of devices to be able to do so. ‘Our task,’ observed de Vries, ‘is to continuously monitor the performance of the SpiroNose and, together with the researchers, expand the applications, such as use with children. The combination with the PCR test, which is also partly performed on people who are assessed negative, is necessary for this.’
The company, financing and regulations
Located at the Bio Science Park in Leiden, Breathomix was founded in June 2018, with a mission to deliver breath analysis using an electronic nose, the SpiroNose, to clinical practice.
The research into breath analysis for Covid-19 was initiated partly at the request of the National Institute for Public Health and the Environment (RIVM), and its start was financed by CbusineZ, a foundation affiliated with health insurer CZ, which aims to stimulate innovation in healthcare. Breathomix has ISO certificates for information security in healthcare.
Beside the SpiroNose, the eNose Company in Zutphen developed the Aenose, which also achieved a high accuracy in a first study. The publication of those results has meanwhile been accepted by a scientific journal. The difference between the two noses is that the test with the Aenose takes a lot longer: five minutes of blowing compared to half a minute with the SpiroNose. The analysis of the Aenose also takes longer.
Profile:
Rianne de Vries is Chief Operational Officer of the Leiden-based company Breathomix. With a background in Technical Medicine (TG) and specialisation in signal analysis, she developed the analysis algorithms behind the BreathBase Platform and the connected SpiroNose. The platform processes real-time measurements of exhaled air, after which automatic analysis takes place to make diagnoses, but also, for example, to predict the effectiveness of therapy. In addition to her position as COO de Vries is completing her PhD research at the lung department of the Amsterdam UMC.
18.03.2021