Seeking biomarkers
On the path to customised medicines
The term 'molecular medicine' has quickly become synonymous with brilliantly revealing images produced by innovative imaging techniques, new biomarkers and contrast agents. At Merck Research Laboratories (MRL) a team of researchers led by Dr Andrew Plump, the firm's Executive Director and Cardiovascular Disease Franchise Integrator, are seeking biomarkers for CVDs, and co-operating with Philips in line with the High Risk Plaque Initiative*, which aims to identify vulnerable and non-vulnerable plaque. (*Reported in EH issue 4/2007). In an interview with Meike Lerner, Dr Plump pointed to two major challenges arising from molecular medicine: Understanding a disease and finding better treatment options. The way forward cannot be found by one company working alone, but by combining knowledge and skills.

ML: What led to the partnership of Philips, Astra Zeneca, BG Medicine and other firms, and what role does Merck play?
Dr Plump: The alliance resulted from a concrete concept that all parties developed together. The aim was not only to leverage the results of each company and compare which result could be useful for which company. The initial thought was: “Let’s come together and put together a research plan that we can push towards the future”. So the High Risk Plaque Initiative was born. This is not a commercial alliance and it is chaired by Dr Valenin Fuster, the gold medal winner of this year’s Congress of the European Society of Cardiology. Merck is collaborating with leading external experts to advance the understanding and treatment of cardiovascular disease. CVD is a leading cause of death worldwide, and there is a great unmet need for new treatments. By working together we see strong synergies and we all believe that real progress in the prevention and treatment of cardiovascular disease is going to come from alliances like ours.
The role Merck plays is to find suitable biomarkers, which have at least two functions: First, they act as contrast agents for the molecular imaging of vulnerable plaque and, in a second step, we can use them as tools in decision making for our early clinical studies in developing pharmaceuticals.
How do you find these biomarkers?
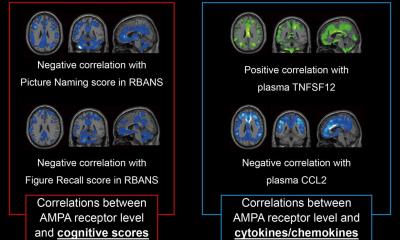
The tradition is to take patient cohorts that include people we think have vulnerable plaque and those who we suggest do not have it, and all their individual blood samples are compared. However, we feel that going directly to the blood has limitations because there is blood in the very non-specific milieu. But looking at the plaque – the disease tissue itself – you can get a deeper insight and receive information that blood does not deliver. So Merck is approaching cardiovascular disease in the same way cancer has been studied – by trying to understand the biology of plaque. Our goal is to go to the disease tissue, compare different kinds of plaque and ask what are the biomarkers more highly expressed in the vulnerable plaque; from there we try to find ways of scaling those to either imaging and our long term project, where we are eager to find something in the tissue that reflects what the disease is really about. We want to find the nature of the plaque and then define a therapy for any individual. We actually have access to literally thousands of plaques, due to a collaboration with a partner company.
One of the things we are doing at MRL, for example, is a series of genomic-based experiments to look at what molecular signatures are present in those plaques.
Our ultimate goal is not to have to go into people and remove plaque, but to turn molecular understanding into some biomarkers and/or diagnostics that we could scale in a non-invasive way. That is where we think molecular imaging will go in our future.
Have you already found biomarkers to differentiate plaque?
To be honest: No, not in the way I’ve described – by looking at the nature of plaque. But we think that, through some of that work, we have made tremendous headway in identifying what we think are very strong candidate markers, which we’ve begun to study. In fact, we are now looking at drug effects on those biomarkers in small clinical studies, because our aim is to make drugs and then turn our discoveries into diagnostics that can be available to physicians and patients worldwide.
What are the next steps from finding a biomarker to produce a pharmaceutical product?
There are two tiers to that question: The first is that, once we believe we have found a biomarker, we can use that in our internal clinical studies by taking the drug and asking what it does to the biomarker. If the biomarker changes in the right direction and we think it does so based on the expected drug effects, we have a drug that is atheroprotective and we start a Phase III trial.
The second tier is a forecast into the future. We hope that we will not have to go through a long, arduous and extremely costly process – ultimately our goal is to try to drive those biomarkers to places beyond decision making, where they are accepted as surrogate markers. So, looking five or ten years ahead, if we do see a change in a promising biomarker that predicts a positive outcome, the biomarker can act as a surrogate marker. And if we can do that, we can push drugs out much more rapidly and will have more drugs available for patients. To present a conclusion: That development will pave the way for the concept everyone is talking about: The concept of more individualised medicine.
30.10.2007