Image credit: Wyss Institute at Harvard University
News • Focused Rotary Jet Spinning
Rapid-production heart valves control blood flow immediately after implantation
Researchers have developed a method for cheaply producing heart valves in the span of minutes that are functional immediately after being implanted into sheep.
The scientists call their method "Focused Rotary Jet Spinning," which they describe as "a cotton-candy machine with a hair dryer behind it." Though long-term in vivo studies are needed to test the valves' endurance, they effectively controlled blood flow for an hour in sheep. The prototype appears in the journal Matter.
"The two big advantages of our method are speed and spatial fidelity," says bioengineer Michael Peters of Harvard University, one of the study's first authors. "We can create really small fibers—on the nanoscale—that mimic the extracellular matrix that heart valve cells are used to living and growing inside, and we can spin full valves in a matter of minutes, in contrast to currently available technologies that can take weeks or months to make."
Cells operate at the nanometer scale, and 3D printing can't reach down to that level, but focused rotary jet spinning can put nanometer-scale spatial cues in there
Kit Parker
Pulmonary heart valves are made up of three partially overlapping leaflets that open and close with every heartbeat. They're responsible for controlling one-way blood flow through the heart; with every beat, they open fully to allow blood to flow forwards, and then close fully to prevent blood from flowing backwards.
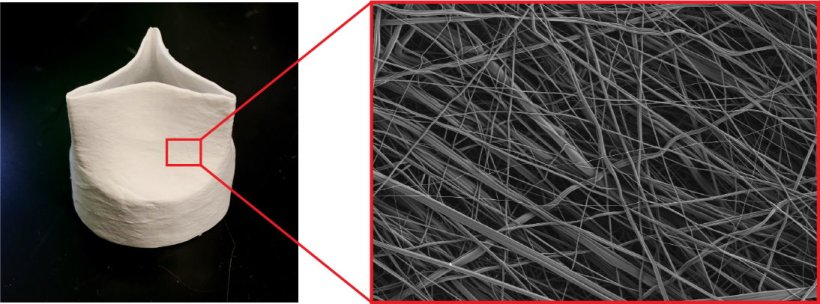
To make the valves, the researchers use air jets to direct liquid polymer onto a valve-shaped frame, resulting in a seamless meshwork of tiny fibers. The valves are designed to be temporary and regenerative: they provide a porous scaffold for cells to infiltrate, build upon, and eventually replace as the polymer biodegrades.
"Cells operate at the nanometer scale, and 3D printing can't reach down to that level, but focused rotary jet spinning can put nanometer-scale spatial cues in there so that when cells crawl up into that scaffold, they feel like they're in a heart valve, not a synthetic scaffold," says senior author and bioengineer Kit Parker of Harvard University. "There's a certain trickery that's involved."
The team tested the valves' strength, elasticity, and ability to repeatedly open and close using a pulse duplicator, a machine that simulates the heartbeat. "A normal heart valve functions for billions of cycles throughout one's life, so they're constantly being pulled and stretched and stimulated," says Peters. "They need to be very elastic and retain their shape despite these mechanical stimuli, and they also have to be strong enough to withstand the back pressures from blood trying to flow backwards."
They also grew heart cells on the valves to test for biocompatibility and to see how well cells could infiltrate the scaffolds. "Valves are in direct contact with blood, so we need to check that the material doesn't cause any thrombosis or obstruction of the blood vessels," says biophysicist Sarah Motta, the study's other first author, who works at Harvard University and the University of Zurich.
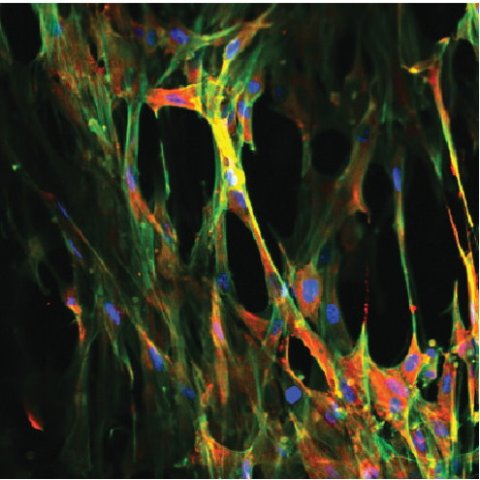
Image credit: Wyss Institute at Harvard University
Finally, the researchers tested the valves' immediate functionality in sheep, who are a good animal model for several reasons—the physical forces inside sheep and human hearts are similar, and sheep hearts also represent an "extreme" environment for heart valves due to sheep's accelerated calcium metabolism, which presents an increased risk of developing calcium deposits, a common complication for heart valve recipients.
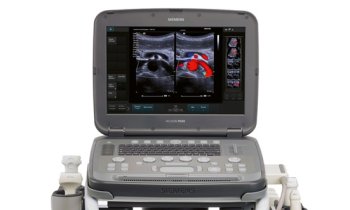
Surgeons implanted the valves into two sheep and monitored their position and function using ultrasound for one hour. Both valves implanted successfully and were immediately functional, but one sheep's valve dislodged after a few minutes—the researchers think this occurred because it was the incorrect size for the animal. In the second sheep, the valve showed good functionality for an hour, and post-mortem analysis indicated that there were no complications in terms of tears or thrombus formation and that cells had already begun to infiltrate and adhere to the valve.
Next, the team plan to test the valves' performance over a longer duration and in more sheep. "We want to see how well our valves function over the scale of weeks to months, and how effectively and quickly the sheep's cells and tissues are actually remodeling the scaffold," says Peters. "It's a long slog to develop something that's going to go into a human patient, and it should be long," says Parker. "You have to do a lot of animals before you put something into a human."
Source: Cell Press/Wyss Institute
08.06.2023