σ=p*r/2h
Physics Law Applied to Improve Left-Sided Heart Failure
Cardiologists from 15 centers in 6 countries have evaluated a new approach for the treatment of Left-Sided Heart Failure under the leadership of Stefan D. Anker, MD, Ph.D., Professor of Innovative Clinical Trials at the Cardiology and Pulmonology Clinic in the Heart Center of the Medical University Göttingen (UMG). The investigators in the clinical evaluation entitled AUGMENT-HF demonstrated that the injection of “Algisyl” hydrogel significantly improved the physical performance and clinical status of patients with advanced heart failure.
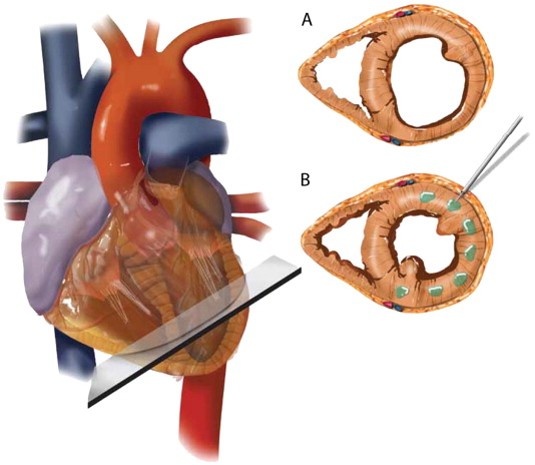

According to Prof. Anker, “the new therapeutic approach is based on the physics law of Laplace which states that the function of a pump – in this case, the heart – depends on wall tension (T) and thus on pressure (p), volume (r), and wall thickness (h) of the pump.” In order to reduce the abnormal tension that is seen in the failing heart, clinicians inject Algisyl-Gel directly into the heart muscle. This modifies the relation of pressure, thickness, and volume leading to a reduction of tension. The therapy was developed by US-based LoneStar Heart, Inc.
Outcomes
The implantation of Algisyl led to a significant and clinically-relevant improvement in treated patients. Peak-VO2, a measure of cardiac function, increased by an average of 18% in comparison to the control patients. Peak-VO2 is a valid and objective parameter to determine the physical capacity and clinical prognosis of patients. Additionally, a six-minute-walk-distance-test was carried out to measure how far patients could walk in a span of 6 minutes. The severely ill patients in the treatment group increased their walking distance by an average of 100 meters representing an enormous improvement from the 300 meters that they walked at the initiation of the study.
Prof. Anker added that “all in all, the outcomes are very positive. 73% of the patients treated with Algisyl indicated that they had a strong to moderate improvement in their quality of life based on a self-assessment.” Cardiologists also evaluated the patients’ health status using the broadly used New York Heart Association classification that grades heart failure from stage I (moderate) to stage IV (severe). Prior to treatment, patients were classified as grade III. One year later, 85% of the patients who received the hydrogel improved to stages I and II.
“This trial raises the hope that a new, effective treatment option for advanced heart failure will be provided in a predictable term. We continue to develop this program with high priority and look forward to additional outcomes” said Prof. Dr. Gerd Hasenfuß, Director of the Cardiology and Pulmonology Clinic and Chairman of the Heart Center at UMG.
Innovative trial design
In the clinical trial, 78 patients were evaluated in a span of four years. The primary efficacy endpoint of peak-VO2 was determined before and at various intervals following treatment with the use of ergometers. Moreover, the investigators used six different test methods to evaluate the severity of the heart failure. Following a process known as double-assessment, mean scores were generated for each patient which markedly raised the reproducibility of testing in all patients treated. According to Prof. Anker, “the double assessment improves the statistical significance of the study even though the total number of patients was not very high.” This method of generating data is not used commonly because the doubling of measures requires a larger effort and use of resources.
Routine use expected in three to four years
It is expected that 3-4 more years will be needed before the new treatment is established in routine medical use. The therapeutic has already received a CE-certification making it compliant with regulations and approved for sale in the European Union. However, before it is used widely, a larger study will be performed in the US to confirm the positive outcomes. Preparations are under way for a trial with approximately 200 patients. The Heart Center at UMG looks forward to participating under the guidance of Prof. Dr. Dr. Stefan Anker.
Original publication:
Douglas L. Mann, Randall J. Lee, Andrew J.S. Coats, Gheorghe Neagoe, Dinu Dragomir, Enrico Pusineri, Massimo Piredda, Luca Bettari, Bridget-Anne Kirwan, Robert Dowling, Maurizio Volterrani, Scott D. Solomon, Hani N. Sabbah, Andy Hinson, Stefan D. Anker. One-year follow-up results from AUGMENT-HF: a multicentre randomized controlled clinical trial of the efficacy of left ventricular augmentation with Algisyl in the treatment of heart failure. European Journal of Heart Failure (2015) doi:10.1002/ejhf.449
Source: Universitätsmedizin Göttingen
05.01.2016