Molecular troublemakers
How proteins prevent communication between bacteria
They may be slimy, but they are a perfect environment for microorganisms: biofilms. Protected against external influences, here bacteria can grow undisturbed, and trigger diseases. Scientists at Kiel University, in cooperation with colleagues at the Hamburg University of Technology (TUHH) in Hamburg-Harburg, are researching how it can be possible to prevent the formation of biofilms from the beginning. On this basis, alternatives to antibiotics could be developed, as many pathogens are already resistant to most commercially used antibiotics.
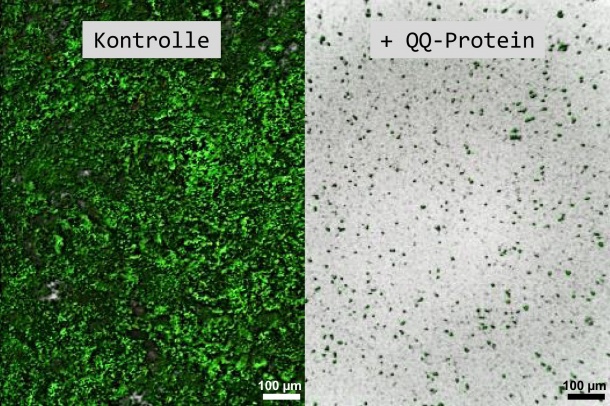

A thin layer floating on water, dental plaque, or slimy black coatings in the washing machine detergent drawer: biofilms originate when cells attach to surfaces, and organise themselves into coordinated three-dimensional consortia, embedded in an extracellular matrix. It becomes problematic when biofilms form on medical devices or implants. Pathogenic bacteria, which trigger deseases, pose a particularly serious threat, as they cannot be treated with normal antibiotics when growing within a biofilm. Therefore: “One way to prevent illnesses is to stop biofilms forming in the first place,” according to Professor Ruth Schmitz-Streit from the Institute of General Microbiology at Kiel University.
In order to coordinate themselves and establish consortia on surfaces, the bacteria must communicate with each other via signal molecules (so-called “autoinducers”). If this communication is disrupted, no biofilm can be formed. This cell-to-cell communication, known as “quorum sensing” (QS), can be influenced by disruptive biomolecules (“quorum quenching” or QQ proteins). “Proteins can break down these signal molecules, or modify them in such a way that they are no longer functional,” explained Schmitz-Streit. Therefore, the goal of the study, financed by the Federal Ministry of Education and Research (BMBF), was to find QQ proteins which disrupt this communication between bacteria as effectively as possible.
In contrast with previous studies, Professor Ruth Schmitz-Streit and Dr. Nancy Weiland-Bräuer, also from Kiel University, concentrated their search on natural environments outside the laboratory. “Because principles which occur in nature have evolved and established over a long time period and are therefore particularly effective,” said Schmitz-Streit. This was demonstrated by the research team by means of a metagenomic approach: they took samples from seawater, from glaciers, but also from jellyfish or from biofilm residue from a washing machine. They extracted the complete DNA from the samples, and used this as a basis to identify proteins with the ability to break down the signal molecules, or render them ineffective.
While doing so, Schmitz-Streit and Weiland-Bräuer determined that the number of QQ proteins which can prevent cell-to-cell communication is extremely high in the marine environmental samples taken – higher than with terrestrial samples. “As the oldest ecosystem, the marine system – including the oceans, water or algae – is incredibly rich in new, undiscovered substances. It offers a huge potential regarding biological activities and QQ mechanisms,” said Schmitz-Streit.
The research group discovered even more: the communication-disrupting protein QQ-2 proved itself to be particularly effective during the investigations. “This protein is very robust and can prevent many different types of biofilms,” explained Weiland-Bräuer. Previous studies focused more on disrupting a particular language of bacteria. “In contrast, the QQ-2 protein is orientated towards a 'universal language', and can disrupt the communication of different bacteria. This makes it a 'general troublemaker'.”
This fundamental research provides important results which may lead to biotechnological and medical applications in future. If the communication of pathogenic bacteria can be deliberately disrupted, it prevents the bacteria from forming biofilms and triggering deseases. In light of the increasing resistance of pathogenic bacteria to antibiotics, the potent effect of natural QQ mechanisms could be an effective approach to the development of medications.
Original publication:
Weiland-Bräuer, N., Kisch, M., Pinnow, N., Liese, A., Schmitz, R.A.: "Highly effective inhibition of biofilm formation by the first 1 metagenome-derived AI-2 quenching enzyme." Frontiers in Microbiology, 13 July 2016. DOI: 10.3389/fmicb.2016.01098; http://journal.frontiersin.org/article/10.3389/fmicb.2016.01098/full
Source: Christian-Albrechts-Universität zu Kiel
01.08.2016