Image source: Pentax Medical
Sponsored • AI assistance for colonoscopy
Ensuring Patient Safety in Endoscopy
Patient safety in endoscopy must be approached from a holistic perspective, through solutions which increase detection rates of abnormalities, increase confidence in the safety of the reprocessing outcome, and control the risk of infection and cross-contamination.
With these important benefits in mind, manufacturers should be continuously working to innovate products to establish solutions that directly tackle patient safety. In this article, Prof. Peter Siersema, Dr. Mira Dreesen, Wouter Meert and Prof. Marco Bruno help to better understand how these innovative solutions ensure patient safety and provide essential benefits to patients and clinicians alike.
Increased detection rates of lesions with Artificial Intelligence
In the fight against colorectal cancer, great strides have been taken to protect patients from undetected abnormalities. However, up to 26% of lesions are still missed in examinations.[1] Endoscopists tiredness or distraction can play a role in complicating the detection.
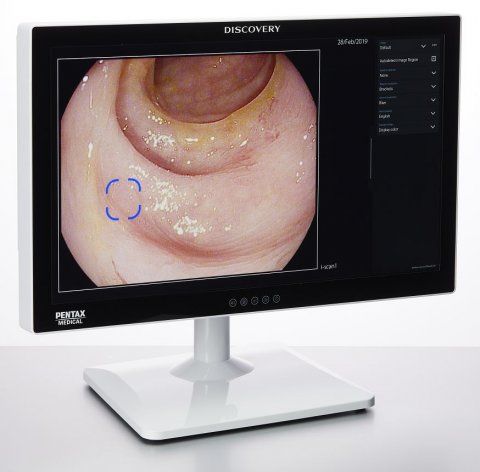
Image source: Pentax Medical
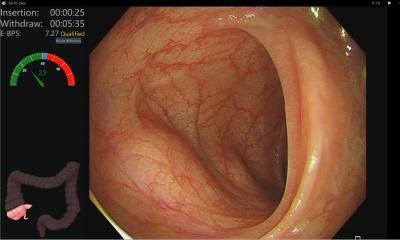
By incorporating artificial intelligence (AI) technology in colonoscopies the procedures become more objective and operator independent. No matter how subtle or unremarkable the lesion, the Discovery™, a new AI solution is endoscopy, is always a focused observer. This innovative solution works to protect patient safety by ensuring consistent detection rates, by reducing the risk of the human factor.
The training of the artificial intelligence was based on a vast amount of expertly curated images. During a procedure, the solution highlights suspicious areas according to insights learned from the database. This database contains thousands of dedicated images of each kind of polyp, annotated by five renowned CRC centers worldwide. By learning from this database, the Discovery™ is able to raise the attention to lesions otherwise difficult to detect.
With patient safety as the greatest priority, this solution could have a positive impact on the 26% of lesions still missed in examinations.[1] Peter Siersema, Professor of Endoscopic Gastrointestinal Oncology at the Radboud University Medical Center Nijmegen, the Netherlands, explains: “Computer-aided detection has been shown to increase the detection of pre-cancerous colorectal lesions. The introduction of the artificial intelligence system opens the door to a new era where endoscopists and Discovery™ jointly reduce healthcare costs by decreasing the prevalence of colorectal cancer.” Prof. Siersema explains that the Discovery™ not only presents the opportunity to improve the patient’s lives, it can also be implemented in hospitals cost-effectively.
Improved confidence in reprocessing outcomes through optimised drying
As patient safety requires a holistic approach, other concerns such as improper reprocessing must be addressed. Flexible endoscopes (FES) are still predominantly reusable devices. During their use, they can become heavily contaminated with the patient’s microbial flora. When FES are not dried properly after reprocessing, microorganisms can proliferate due to residual moisture and represent a source of infection for subsequent examined patients.[2]
As effective drying and storage procedures are crucial to prevent post-endoscopic infection, this is an important aspect of patient safety.[3] The PlasmaTyphoon guarantees a dry endoscope in one to five minutes (the drying time depends on the endoscope type), and storage up to 31 days in a fully controlled environment.[4] [5] After the completion of the drying process, the single use PlasmaBag comes into play: Plasma, containing ozone molecules, is insufflated into the bag ensuring the dry and disinfected state of the endoscope is maintained due to biocidal effect of the ozone.
By using the PlasmaTyphoon and PlasmaBag system, confidence in the safety of reprocessing outcomes can be improved. Once the endoscopes are dried and stored using this innovative solution, cross contamination and recontamination risks from handling and transportation are minimised. Dr. Mira Dreesen, hospital pharmacist at the University Hospital Leuven, discusses her experience: “We always perform routine laboratory testing to check the endoscopes. Currently using a multifactorial process, including the PlasmaTyphoon and PlasmaBag system, we see improved test results. Considering the importance of the drying phase, this leads us to believe that the PlasmaTyphoon helps us to achieve the drying process faster and more cost effectively. Although the University Hospital Leuven has only recently implemented the PlasmaTyphoon in our hospital, we expect our results will be become even better because of the use of this device, in the near future.”
Wouter Meert, Process Manager at the University Hospital Leuven, goes into greater detail regarding the ways in which the PlasmaTyphoon and PlasmaBag system support safe endoscope transportation whilst at the same time reducing the need for repeated reprocessing. Wouter Meert explains: “We have two treatment areas for FES within the hospital, due to many patient needs in different wards we must be able to store FES one month after reprocessing, so that scopes can be ready for use for urgent procedures. A major benefit we see using the PlasmaBag is that you have a covered scope, which can be easily put in closed trolleys and transported around the whole hospital.” As endoscopes can be confidently and completely dried using the PlasmaTyphoon, and stored in a fully controlled environment free of moisture, dust or contaminants using the PlasmaBag, this innovative solution instills greater confidence that endoscopes will be safe for the next patient.
Minimised risk of contamination with disposable elements
Innovative solutions for proper cleaning of endoscope channels are critical to minimising the risk of contamination, and safeguarding patient safety. Concerns of cross-contamination arose after increasing incidences of Carbapenem-resistant Enterobacteriaceae (CRE) and other infections. These infections may be linked to improper cleaning and/or disinfection of the duodenoscope’s elevator mechanism. Prof. Marco Bruno, Director of Endoscopy in the Department of Gastroenterology and Hepatology at the Erasmus Medical Center in Rotterdam explains: “A solution to the risk of infection, is to modify current endoscopes so that they have disposable parts. Especially the parts that are most susceptible to contamination. Another solution would be to have fully disposable endoscopes. This of course introduces questions about pricing and the environmental impact, of which need to be solved by manufacturers and medical facilities before this solution can be used in daily practice.”
Technical solutions can contribute to the development of a safer device. For example, the DEC™ Video Duodenoscope ED34-i10T2, aims to address one of the main areas of concern in terms of potential infection, the elevator, and in turn responding to the need for enhanced patient safety in endoscopy. It is a unique and innovative advancement in cleaning capabilities for infection prevention and control, with the single-patient use, sterile, disposable elevator cap (DEC™). The DEC™ allows simplified reprocessing and increased cleaning capability, thus helping to reduce the risk for cross-contamination. With this solution there is greater reprocessing efficiency, 35% reduction in distal end reprocessing steps due to better access for cleaning and disinfection as well as disposability of the elevator.[6]
With patient safety as the greatest priority, manufactures should offer innovative solutions that protect patients and provide clear benefits to clinicians.
References
3 Kovaleva J. Endoscope drying and its pitfalls. J Hosp Inf 2017:97;4:319 – 328
4 Evaluation of the ability of a storage system (plasmabiotics) to maintain the microbiological quality of heat sensitive endoscope. Report by Biotech-Germande April 2017.
5 Storage times are subject to local regulations.
6 * In comparison to the standard duodenoscopes of the major manufacturers. Source: PENTAX Medical internal benchmarking.
Source: Pentax Medical
19.11.2020