Image credit: Adam Feinberg and Andrew Hudson, Carnegie Mellon University
News • Freefrom Reversible Embedding of Suspended Hydrogels
A 'FRESH' way to 3D-print tissues and organs
Research into 3D bioprinting has grown rapidly in recent years as scientists seek to re-create the structure and function of complex biological systems from human tissues to entire organs.
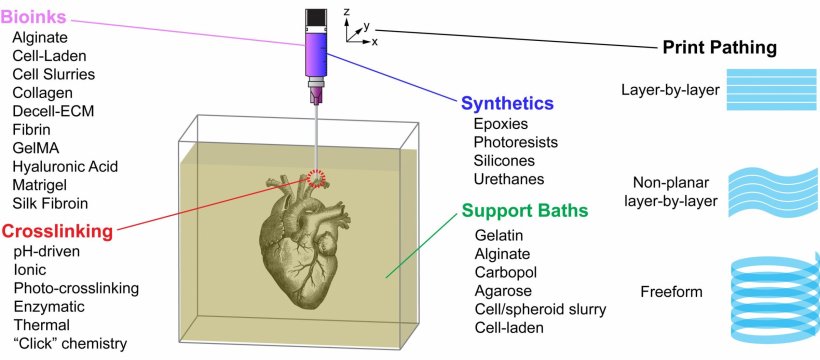
The most popular 3D printing approach uses a solution of biological material or bioink that is loaded into a syringe pump extruder and deposited in a layer-by-layer fashion to build the 3D object. Gravity, however, can distort the soft and liquid bioinks used in this method. In APL Bioengineering, by AIP Publishing, researchers from Carnegie Mellon University provide perspective on the Freefrom Reversible Embedding of Suspended Hydrogels (FRESH) 3D bioprinting approach, which solves this problem by printing within a yield-stress support bath that holds the bioinks in place until they are cured.
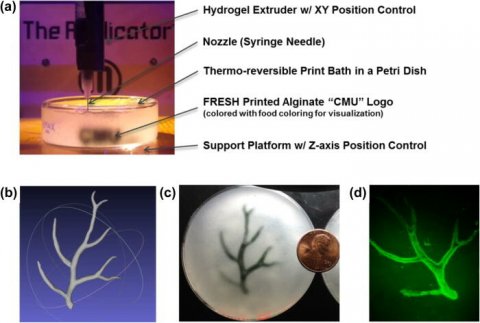
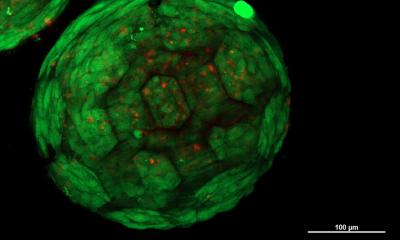
Image adapted with permission from Hinton et al., Sci. Adv. 1(9), e1500758 (2015). Copyright 2019 Authors, licensed under a Creative Commons Attribution (CC BY 4.0) license.
Until now, the distortion of bioinks, which results in a loss of fidelity, had presented a challenge to fabricating functional adult-sized tissues and organs and is a barrier to the long-term goal to supplement the limited donor supply for transplant. Consequently, most 3D-bioprinted tissue constructs to date have been relatively small when compared to the tissues or organs they are intended to replace. “Our goal is to be able to FRESH 3D-print complex 3D tissue and organ models out of a wide range of biocompatible hydrogel and cell-laden bioinks,” said author Adam Feinberg.
The FRESH technique embodies several unique aspects. First, a support bath enables the printing of cells and bioinks that maintain their position as they cure, while still allowing for the movement of the extrusion needle. The FRESH support bath also provides an environment during the printing process that maintains high cell viability. FRESH provides the ability to work with the widest range of bioinks of any 3D-bioprinting method. Finally, it uses a nondestructive print release by warming up the ink to 37 degrees Celsius to gently melt the support bath at body temperature.
Since it was developed in 2015, FRESH has been adopted by many research labs, for projects such as the FRESH printing of nanocellulose, conductive hydrogels, scaffolds for stem cell grown, and ventriclelike heart chambers composed of beating heart muscle cells. The researchers have recently initiated a number of studies to FRESH 3D-print skeletal muscle, including controlling muscle architecture and regenerating muscle tissue after volumetric muscle loss.
Source: American Institute of Physics / AIP Publishing
17.02.2021