News • Biomaterial research
These cellular insights could make our bones heal faster
Most of us don’t think about our teeth and bones until one aches or breaks. A team of engineers at Washington University in St. Louis looked deep within collagen fibers to see how the body forms new bone and teeth, seeking insights into faster bone healing and new biomaterials.
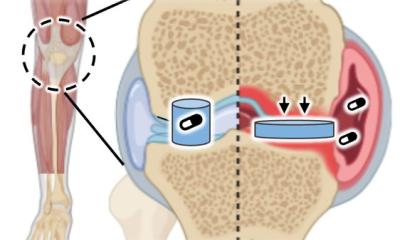
Young-Shin Jun, professor of energy, environmental & chemical engineering in the School of Engineering & Applied Science and director of the Environmental NanoChemistry Lab, leads a team of experts in nucleation, the initial step in forming a solid phase in a fluid system. While nucleation of minerals in bone and teeth is not well understood, researchers do know that bone minerals form inside of collagen, the main protein found in skin and other connective tissues. Jun and Doyoon Kim, a doctoral student in her lab, studied how miniscule gaps in collagen’s fiber structure facilitate the nucleation of calcium phosphate, which is necessary for bone formation and maintenance.
The findings, recently published in Nature Communication, provide a new view into the current theory of calcium phosphate nucleation in a confined space.
To observe nucleation in a collagen gap — about 2 nanometers high and 40 nanometers wide — the team studied calcium phosphate nucleation with in situ small-angle X-ray scattering at the Advanced Photon Source in Argonne National Lab. They found that without an inhibitor, nucleation initially took place outside of the collagen gap. When they added an inhibitor, the process occurred mainly within the collagen gap. Jun said the extremely confined space in the collagen gap allows calcium phosphate to form only along the length of the gap and minimizes surface interactions with the gap sidewalls. In other words, the topography of the collagen gap decreases the energy cost and enables nucleation.
If we can tweak the chemistry and send signals to form bone minerals faster or stronger, that would be helpful to the medical field
Young-Shin Jun
“When we understand how new bone forms, we can modulate where it should form,” Jun said. “Previously, we thought that collagen fibrils could serve as passive templates, however, this study confirmed that collagen fibrils play an active role in biomineralization by controlling nucleation pathways and energy barriers. If we can tweak the chemistry and send signals to form bone minerals faster or stronger, that would be helpful to the medical field.”
While this study focused on the biological aspects of nucleation, Jun said an advanced understanding of nucleation in confinement also applies to chemical engineering, materials science and environmental science and engineering. “Confined space is a somewhat exotic space that we have not explored much, and we are always thinking about new material formation without any limitation of space,” Jun said. “However, there are so many confined spaces, such as pores in geomedia in subsurface environments or in water filtration membranes, where calcium carbonate or calcium sulfate form as scale. This paper is a snapshot of one health aspect, but the new knowledge can be applied broadly to energy systems and water systems.”
Source: Washington University in St. Louis
08.04.2018