The ESPOIR Study
Professor Axel Haverich and team at the Clinic for Cardiothoracic, Transplant and Vascular Surgery in Hanover Medical School (MHH) have been carrying out research into decellularised heart valves for over 15 years. They trialled a procedure – initially in the laboratory and in animal experiments – which does not cause tissue rejection, is hoped to last a lifetime and, in the case of children, even to grow with the patient.

Since January 2012 this procedure has been tested at eight Paediatric Cardiology Centres based in Moldova, Italy, France, Belgium, The Netherlands and Great Britain in the context of the European Clinical Study of Regenerative Heart Valves (ESPOIR) under the leadership of the MHH.
The European Union will support this regenerative medicine research project with €5.2 million until the end of 2016. At the heart of the study is the consolidation of research results from the individual cardiology centres into a joint database. Only through long-term follow-up of the patients examined along with a cross-centre evaluation will it be possible to confirm the success of the new procedure.
In May 2002, two children in Moldova where the first to he implanted with a decellularised heart valve. This not only launched the promising medical procedure but was also the beginning of a protracted, sometimes public debate. Finally, after 11 years, the Paul- Ehrlich-Institute recently authorised decellularised heart valves as pulmonary valve replacements, confirming the success of the treatment so far. ‘We have now implanted one hundred decellularised heart valves and haven’t had to remove a single one,’ said Dr Samir Sarikouch, private docent, clinical trial leader and senior physician at the Clinic for Cardiothoracic, Transplant and Vascular Surgery Clinic in Hanover.
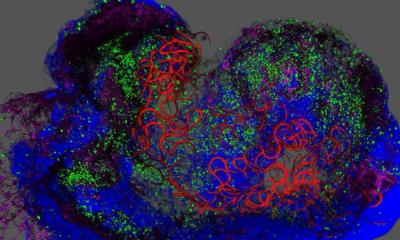
The new procedure uses donated human heart valves, which are supplied by the European Homograft Bank (EHB) and the German Society for Tissue Transplantation gGmbH (DGFG). As a sufficient supply of tissue donations is what the procedure relies upon, the study also aims to improve cooperation between tissue banks across Europe. The donor’s protein is completely removed from the valves in a special laboratory. ‘In indicathe case of the so-called homografts only the collagen of the supporting tissue remains as a matrix, which is transplanted and then populated with the recipient’s cells. These cells then progressively regenerate the body’s own tissue. Depending on the recipient’s age this process can take months or even years, but it works,’ Dr Sarikouch pointed out. Removing the protein from the transplanted tissue prevents the recipient’s immune system from detecting it as foreign and so avoids the onset of a rejection process.
Dr Sarikouch and colleagues could demonstrate this only recently, in a study with children and adolescents. Whereas the implantation of conventional mechanical heart valves requires lifetime medication to thin the patient’s blood, and biological (human or animal) heart valves need to be replaced after eight to ten years, the new procedure has none of those disadvantages. ‘There are strong indications that these heart valves will be much more durable than previous valves that were not decellularised – and even indications that they can grow with the patient,’ he said. ‘To monitor this, the patients treated have to be closely observed for years and decades.’ As children require smaller homografts than adults, cutting larger heart valves to size for smaller organisms has been trialled in animal experiments, with good results.
MRI has become established as the imaging procedure of choice because it is the only procedure that does not cause children any permanent damage through X-ray radiation during the annual examinations.
Over the years, these examinations generate vast amounts of image data, which not only need safe storage but also collation in Hanover for the European study. ‘We’re very pleased that T-Systems recognised this market potential, and is developing a system that consolidates this data flow – the pseudonymised MRI images as well as the study data. The trick is to design the system so flexibly that secure access to the data will still be possible in 25 years’ time. Ensuring this is obviously easier for a market leader than for a smaller company, such as an IT spin-off from a university,’ Dr Sarikouch explains. Not only the longevity of data access but also speed, flexibility and, in particular, security, are important points that T-Systems must address.
In the future, it will be possible to access the corresponding MRI data sets in parallel to an analysis of case report from data, without the need to download them individually from the PACS. ‘The data is centrally stored and we can sort and process it as we see fit. We can view the filtered data sets immediately and completely and can commence our work. It may sound trivial, but previously there was no such procedure available,’ he explains.
In parallel, Dr Sarikouch will also have the CDs sent to him from the participating centres during the test phase. As is so often the case, the devil is in the detail.
He assumes that any teething problems with the solution will be resolved within a year (i.e. by 2015) and that the system will meet all expectations during trials.
Profile:
After gaining a medical degree in Essen and doctorate at RWTH Aachen University, Dr Samir Sarikouch specialised in paediatric surgery and cardiology, practising at the Heart Centre Duisburg and the Heart and Diabetes Centre in Bad Oeynhausen, among others. Since 2008 he has been a senior physician at the Department for Cardiothoracic, Transplant and Vascular Surgery as well as Head of Clinical Research & Biostatistics at Hannover Medical School. He is a specialist in paediatric surgery, general paediatrics, paediatric cardiology, and paediatric intensive care and in treating adults with congenital heart disease.
29.10.2013