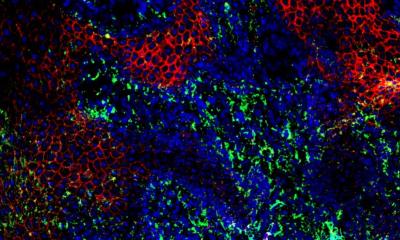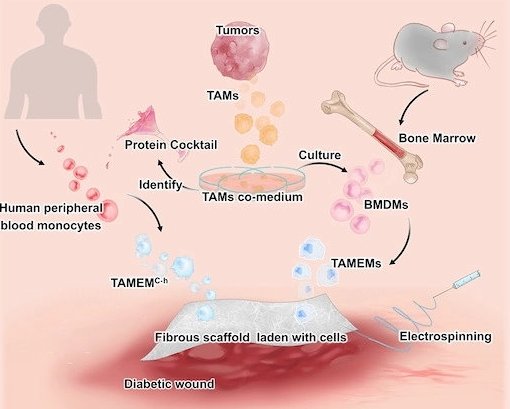
Image source: Mu et al., EMBO Molecular Medicine 2022 (CC BY 4.0)
News • Regenerative medicine
Tapping into a tumor's power to heal non-healing wounds
Scientists have discovered a way to train healthy immune cells to acquire the skills of some tumor cells for a good purpose: to accelerate diabetic wound healing.
This remarkably promising finding, recently published in EMBO Molecular Medicine, may open up a brand new avenue for regenerative medicine.
Diabetes affects more than 100 million people worldwide, and its most severe complication—diabetic foot ulcers (DFUs)—causes an amputation every 30 seconds on average. One of the fundamental reasons behind the non-healing status of DFUs, compared with common cutaneous wounds, is the dysfunction of a mixed group of immune cells called macrophages. These cells change their functions to coordinate wound healing in different stages but fail to do so under a diabetic pathology. Previous attempts to reprogram the macrophages have proven suboptimal.
The collaborative team, led by Professor Chunming Wang at the University of Macau and Professor Lei Dong at Nanjing University, are inspired by tumor-associated macrophages (TAMs), which play essential roles in driving tumor development by secreting factors that promote blood vessel formation and inhibit immune attack. Such features perfectly fulfill the requirements for diabetic wound healing. They boldly hypothesized that TAMs could "pass on" these features to non-tumor macrophages through co-culture, in a way that TAMs influence other cells in cancer growth.
The team designed and optimized protocols to verify this hypothesis, finding that normal macrophages derived from mouse bone marrow can gain a new set of pro-regenerative functions after co-culture with TAMs. When transferred to the wound bed in diabetic mice, these cells potently induced cell proliferation, resolved inflammation and orchestrated vasculature in the typically non-healing wounds. Furthermore, gene analytical tools, one of which is named single-cell RNA sequencing, revealed some surprising findings. These TAMs-educated macrophages (TAMEMs) are distinct from known phenotypes (typically simplified as M1 or M2 in biological terminology). In short, these cells exhibit unique skills after training that appropriately fulfill the demands of diabetic wounds.
The team made further strides by trying to dissect the secret of TAM's power—e.g., identifying the multiple factors that equip normal macrophages with these reparative capabilities. Eventually, they reconstituted a nine-factor cocktail and tested its efficacy on—this time, human—monocytes, leading to a desirable outcome. Furthermore, this test totally abandons any tumor-derived components (including TAMs), representing the imminent translational potential for a clinical trial.
Professor Lijian Hui, an expert from the Shanghai Institute of Biochemistry and Cell Biology, China, highly appraised the creativity and translational potential of this work. According to Hui, this work is very creative in recapitulating the characteristics of TAMs for diabetic wound healing. It is also significantly sets a good example of elucidating the mechanisms of actions of therapeutic cells (TAMEMs, in this case), which is often overlooked in developing cell therapy approaches. Hui expects the team to further their work to understand the variances and heterogeneity of the engineered macrophages, tackling the challenges in quality control and accelerating the preclinical tests.
Source: Nanjing University School of Life Sciences
04.01.2023