Illustration: Empa
News • Finding the fibrils
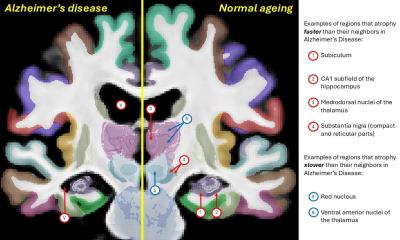
The “superspreaders” behind Alzheimer's disease
In dementia diseases such as Alzheimer's, incorrectly folded proteins accumulate in the brain. Empa researchers have now resolved a particularly active species of protein fibrils with unprecedented precision.
The formation of potentially toxic molecules on the surface of protein fibrils was studied from early to late stages spanning over a period of hours. The researchers recently published their results in the journal Science Advances.
The treatment of dementia disorders such as Alzheimer's is still one of the greatest challenges facing modern medicine. In the course of neurodegenerative diseases, certain proteins such as the amyloid β protein accumulate in the brain. They are suspected of being linked to the development of the disease, which is why they are considered a promising target for therapeutic approaches.
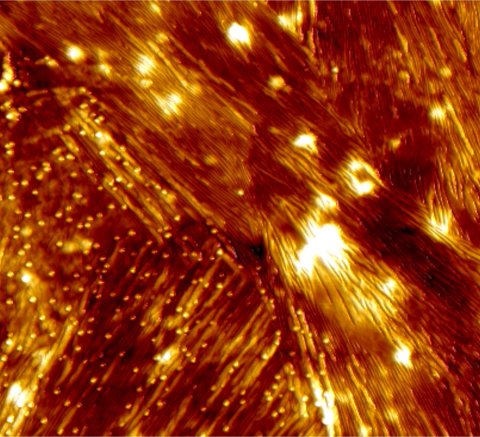
Image source: Empa
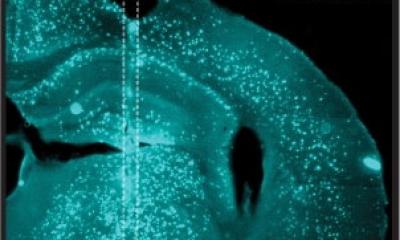
It is already known that the misfolded proteins clump together to form fiber-like structures. However, it is not yet fully understood how these fibrils are formed. Now a team led by Empa researcher Peter Nirmalraj from Empa's Transport at Nanoscale Interfaces laboratory and scientists from the Irish University of Limerick have been able to show how the process takes place using a particularly powerful imaging technique. The special thing about it: Some of the nanometer-thin fibrils apparently ensure the spread of the disease in the brain tissue and are therefore referred to as “superspreaders”.
This peculiar subspecies of protein fibrils caught the researchers' attention because of its unusual properties: The edges and the surfaces of the so-called superspreader fibrils show a particularly high catalytic activity. New protein building blocks accumulate at these highly active sites. As a result, new, long-chain fibrils form from these nucleation sites. The researchers assume that these second-generation fibrils eventually spread and form new aggregates in the brain.
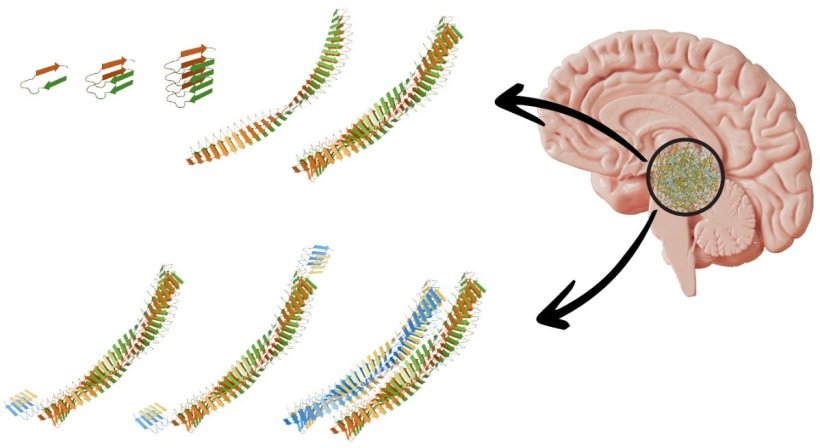
Illustration: Empa
The chemical composition of the misfolded amyloid β protein is known. The mechanism of how protein building blocks come together to form second-generation fibrils, as well as their shape and structure, was previously unclear. “Conventional methods, such as those based on staining techniques, could alter the morphology and adsorption site of the proteins so that they cannot be analyzed in their natural form,” says Nirmalraj.
This work brings us another step closer to better understanding how these proteins spread in brain tissue of Alzheimer's disease
Peter Nirmalraj
The technique the Empa researcher used in this new study is different: The proteins are analyzed unchanged in a salt solution, which comes much closer to the natural conditions in the human body than is the case with conventional methods. With the high-resolution atomic force microscope, the fibrils, which are less than 10 nanometers thick, can be photographed with unprecedented precision at room temperature. The researchers were able to follow the process of fibril formation in real time, from the first moments to the following 250 hours. The analyses were then compared and supplemented with molecular model calculations. This allowed the fibrils to be classified into subpopulations such as “superspreader” based on their surface structures. “This work brings us another step closer to better understanding how these proteins spread in brain tissue of Alzheimer's disease,” says Empa researcher Nirmalraj. He hopes that this will ultimately lead to new ways to monitor disease progression and diagnostic procedures.
The study was funded by the Dementia Research Switzerland - Synapsis Foundation.
Source: Empa
28.10.2024