© Medicaroid
Article • New technologies, old limitations?
Robot-assisted surgery in Europe: high tech between scalpel and legislation
The use of surgical robots offers a wide range of possibilities, but in Europe the technology is often unable to reach its full potential. Dr. med. Christoph Wandhöfer explains why.
Guest article from Dr. med. Christoph Wandhöfer, Medicaroid Europe
Robot-assisted surgery is considered one of the most promising developments in modern medicine. It enables precise, minimally invasive procedures that can speed up recovery. But its true potential goes far beyond that: technologies such as telesurgery—remote surgery performed by experienced surgeons across large distances—are bringing more widespread, specialized care within reach.
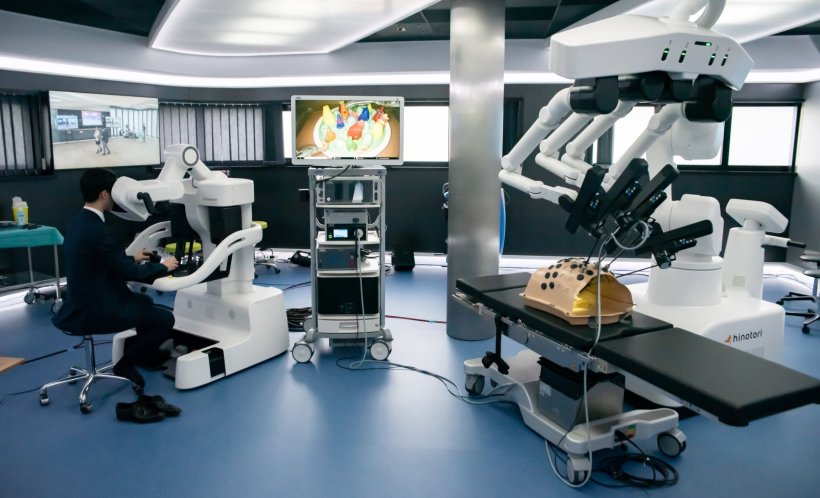
Despite increasing technological maturity, this vision still faces practical hurdles in Europe. Ethical and regulatory frameworks, approval processes, and national differences are the main barriers to integrating these technologies into routine clinical practice. One example is the hinotori robotic assistance system from Japan, which illustrates the tension between technological progress and legal certainty in a highly sensitive field.
The regulatory bottleneck: CE certification under the MDR
Before such systems can be used in Europe, they must obtain CE marking in accordance with the Medical Device Regulation (MDR). In effect since 2017, this regulation is considered one of the world’s most stringent frameworks for medical devices. It categorizes devices by risk level; due to their invasive application, surgical robots are classified in a high-risk category that requires particularly comprehensive evidence of technical, clinical, and application-specific safety.
A key aspect here is the “intended use”—that is, the precise medical application of the product. The manufacturer must specify whether the system is to be used in, gynecology, urology, or general surgery, for example, and must provide targeted evidence that the system functions safely within that particular field. Several approaches can be used for this purpose, ranging from cadaver studies and clinical trials to comparisons with internationally established systems.

© Medicaroid
In practice, however, implementation is often challenging. “The current EU MDR from 2017 is a document of over 170 pages, including numerous annexes that sets out the requirements for all medical devices. Nonetheless, there are always unresolved questions when it comes to the details,” explains Dr. Christoph Wandhöfer from Medicaroid Europe.
One reason for this lack of clarity is that the process begins with the selection of a “notified body”—an independent certification organization such as TÜV Nord or TÜV Süd—which is responsible for evaluating and certifying the product. The number of notified bodies was significantly reduced after the previous regulatory directive, the MDD, was repealed and replaced by the EU MDR. Those that remain must realign themselves and adapt to the significantly increased requirements for technical documentation.
“As a result, the certification process tends to proceed in cycles: documents are submitted, questions are raised, and adjustments and corrections are made until the notified body's requirements are finally fulfilled,” says Dr. Wandhöfer. In addition, many products that were already approved under the previous directive must now undergo recertification. All of this leads to bottlenecks and, in some cases, lengthy waiting periods. It is difficult to predict how long the process will take—it could be six months or a year and a half.
When innovation has no reimbursement code
Another obstacle to widespread clinical use is a lack of financial integration. In Germany, robot-assisted procedures cannot currently be reimbursed, regardless of the added value for patients or the cost incurred by hospitals. This is offset by high investment, organizational, and training costs.
Countries like Poland have already introduced initial reimbursement models to provide targeted incentives for modern technologies. These models could also serve as a reference for other countries—especially if robotic procedures are expected to help reduce complications and shorten hospital stays in the long term. “What is needed are harmonized regulations across national borders,” says Dr. Wandhöfer.
Remote surgery: regulatory pitfalls
This patchwork of organizational, legal, and ethical frameworks also complicates the implementation of one of the most exciting and media-effective potentials of surgical robotics: remote surgery.
The idea of making medical excellence available regardless of location is as old as robotics itself. The U.S. military, in particular, invested in the development of surgical capabilities for battlefield conditions decades ago—thus laying the foundation for robotic surgery.
There are no clear regulations governing connectivity in the operating room—who is liable in the event of a network failure, how backup systems must be organized, or what minimum standards apply
Christoph Wandhöfer
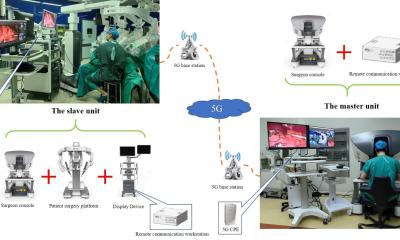
Today, with the rollout of 5G and the prospect of 6G, much is technically feasible, and countries such as China have already gained experience with remote surgery—often with targeted government support and specially developed network infrastructure.
In Europe, however, telesurgery remains a vision for the future: there are no clear legal frameworks. “There is no unified legal basis that permits or regulates cross-border remote surgery,” says Dr. Wandhöfer. Key issues such as liability, data protection, medical ethics standards, and responsibilities in emergency situations remain unresolved.
Cybersecurity also continues to be a source of uncertainty: “You can't simply order a stable connection for a surgical procedure from a telecom provider,” Dr. Wandhöfer explains. “There are no clear regulations governing connectivity in the operating room—who is liable in the event of a network failure, how backup systems must be organized, or what minimum standards apply.” Many of these aspects would need to be addressed in both national and European regulatory frameworks.
Conclusion: recognize opportunities, remove obstacles
Regardless of the future potential of remote surgery, robot-assisted procedures already offer concrete benefits for patients and healthcare systems—through more precise interventions, shorter hospital stays, and potentially fewer complications.
Nevertheless, the barriers to widespread adoption remain high. In many European countries, high investment and maintenance costs contrast with a lack of financial integration. This is the case despite the existence of initial financing models from manufacturers designed to facilitate acquisition. Only if these structural and financial hurdles are systematically addressed can robotic surgical systems realize their full potential and play a central role in a sustainable, efficient, and patient-centered healthcare system.
12.06.2025