News • Study program
Overcoming the hurdles in translational medicine through education
The School for Translational Medicine and Biomedical Entrepreneurship (sitem-insel School) in Bern, Switzerland, starts for the third time its Study Program in Translational Medicine and Biomedical Entrepreneurship.
The program offers continuing education to specialists in industry, hospitals and academia in the field of translational medicine and biomedical entrepreneurship, expertise highly needed in both industry and universities. Participants will acquire the know-how to initiate and implement the translation process of products from the stage of development in industrial or research institutions to clinical applications with the goal of their commercialization. The focus is on medicinal products as well as diagnostic and therapeutic medical devices.
The program addresses professionals from industry, hospitals and academia that are active in the medical sector and interested in translational medicine and biomedical entrepreneurship. It is conceptualized as extra-occupational program that can be reconciled with the usual professional work. The study program includes a blended learning concept which means that remote e-learning will be complemented by concept lectures, peer learning and interactive discussions with experts. Having only a limited number of in-class courses allows for large flexibility while at the same time permitting participants to profit from the experts’ expertise.
The program is highly modular, thus allowing individual learning approaches. It consists of a prerequisites section (e-learning) and six modules (blended learning). It is also possible to book single modules or groups of modules.
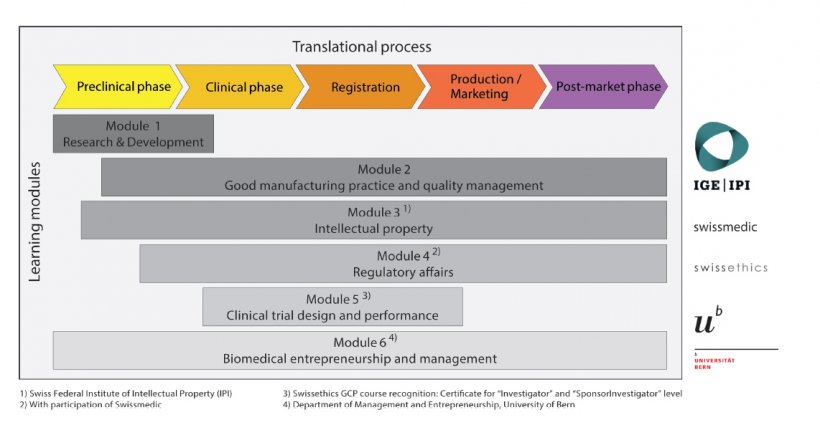
Learning modules
- Prerequisites
The prerequisites section is organized as e-learning and offers participants the possibility to refresh their knowledge according to their individual needs, covering the basics of biopharmacy, pharmaceutical technologies, medical technologies, OMICS technologies and biostatistics and epidemiology.
- M1 Research and Development (4 ECTS)
Module 1 revises basic principles related to the discovery and development of medical devices and medicinal products.
- M2 Good Manufacturing Practice and Quality Management (5 ECTS)
Module 2 focusses on how to ensure that all activities linked with a translational process maintain the desired level of excellence and quality required by regulatory agencies.
- M3 Intellectual Property (3 ECTS)
Module 3 is dedicated to different types of Intellectual Property and legal aspects that are crucial for the successful commercialization of medical devices and medicinal products.
- M4 Regulatory Affairs (5 ECTS)
Module 4 focuses on the approval process for bringing innovative products to the market and describes the role of regulatory agencies, national and international authorities and notified bodies along the translational pathway.
- M5 Clinical Trial Design and Performance (6 ECTS)
Module 5 considers key characteristics of clinical trial design and conduct. Participants learn about the prerequisites for such scientific studies, the importance of understanding the pathophysiology of the underlying diseases, the definition of quantifiable endpoints by clinicians as well as data management and statistics.
- M6 Biomedical entrepreneurship and management (13 ECTS)
Module 6 concentrates on aspects of entrepreneurship with special focus on medicinal products and medical devices including leadership in multidisciplinary teams, project management, business administration and financial aspects that are needed for the successful commercialization of medical devices and medicinal products.

Admission Requirements
Certificate of Advanced Studies (CAS), Diploma of Advanced Studies (DAS): Participants should have a Bachelor’s, Master’s or higher degree in life sciences, medicine, pharmacy, engineering science, natural sciences or equivalent. Participants are welcome to bring a proposal for an own translational medicine project on which they will work during their master thesis; however, this is not a requirement.
Fees
The fee for the Master of Advanced Studies (MAS) in Translational Medicine and Biomedical Entrepreneurship is CHF 31’500.-, for the DAS programs CHF 23’100.- and for the CAS programs CHF 12’600.-. The fee for single modules is between CHF 3’150.- and CHF 6’300.-, depending on the size.
Scholarships
The School grants scholarships for innovative projects.
Additional Information
The MAS study program (60 ECTS) starts in September 2019 and is designed for a minimum duration of two years but can be extended according to individual requirements. DAS (30 ECTS) and CAS (15 ECTS) certificate programs or single modules (3-5 ECTS) can be started later. The course language is English.
In-class courses are held at the Swiss Institute for Translational and Entrepreneurial Medicine, sitem-insel, in Bern, Switzerland. Participants will be registered at the University of Bern.
Registration and Contact
Mail: school@sitem.unibe.ch
Phone: +41 31 664 6400
14.08.2019