Source: Merck
News • Collaboration with Mammoth Biosciences
Merck to scale up new SARS-CoV-2 Test
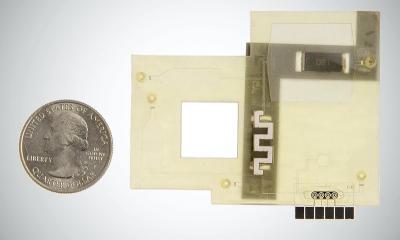
Merck announced a collaboration with Mammoth Biosciences Inc. for the development, scale-up and commercial production of Mammoth’s CRISPR-based SARS CoV-2 diagnostic test. Mammoth’s high-throughput systems will be compatible with both nasal swab and saliva samples and are targeting approximately 1,500 tests per 8-hour shift with minimal user interaction. Mammoth Biosciences plans to submit the assay for FDA Emergency Use Authorization later this year.
“This collaboration will allow us to help Mammoth with the development and production of their new SARS-CoV-2 test, which, once approved by the FDA, will increase testing capacity here in the U.S. at the scale needed to combat this pandemic,” said Jean-Charles Wirth, head of Applied Solutions, Life Science, at Merck. “Merck is helping to advance the future of testing at scale. This is an important step in detecting Covid-19 quickly.”
The Life Science business of Merck will serve as the contract manufacturer of the DETECTR BOOST SARS-CoV-2 Reagent Kit, which will use standard, automated liquid handling equipment to allow rapid processing of patient samples. With this new test, Clinical Laboratory Improvement Amendments labs in the U.S. will be able to significantly improve capacity to regularly perform testing. According to Mammoth, the new test is expected to use a CRISPR-based workflow that reduces the time required for testing while maintaining the gold standard accuracy of Polymerase Chain Reaction expected by lab operators.
“Leveraging the proven manufacturing and product leadership of the Life Science business of Merck is critical to Mammoth delivering a CRISPR-based diagnostic solution to labs,” said Janice Chen, Ph.D., co-founder and chief technology officer of Mammoth Biosciences. “We have the opportunity to make a tremendous positive impact on the pandemic by enabling the further scale-up of our nation’s testing capacity.”
Mammoth recently secured funding from the National Institute of Health’s RADx program to scale its CRISPR-based testing workflow.
The Applied Solutions business unit, part of the Life Science business of Merck, offers a broad range of products and services for clinical and diagnostic testing laboratories as well as the food and beverage industry. It provides technologies that streamline processes, lower costs and deliver consistent, reliable results. The production will take place at the business’ Life Science Center in St. Louis, Missouri, USA.
Merck is committed to the fight against Covid-19. The company’s products are being used by scientists around the world to develop more than 50 vaccine candidates, more than 35 testing solutions and more than 20 monoclonal antibodies, plasma products and antivirals to combat the virus.
Source: Merck
30.10.2020