Firm imposes press embargo
Another patient in the final stages of heart failure has received an artificial heart at Nantes UniversityHospital Centre, according to Carmat, the manufacturer of the device.
Report: John Brosky
Carmat announced, in a press release, its active recruitment of two more patients to complete its first-inhuman clinical trial of the artificial heart against a primary endpoint of 30-day survival with secondary criteria for assessing the impact of the reinvigorated blood supply from the mechanical heart on internal organs. ‘We warmly thank, particularly today, the experienced team at CHU-Nantes,’ stated Carmat CEO Marcello Conviti. ‘Passing this step was made possible thanks to their confidence as well as that of our participants, partners and investors.’ The company was forced to issue the press release after the newspaper Liberation reported a second implantation.
Although the news leak was reported by all French media, none could report any further detail about the patient’s condition. The company imposed a news blackout regarding implantations after the media circus that followed the news of the first implantation and the subsequent death of the patient 74 days later. The company’s stock price jumped on the first news and then fell dramatically with the death of the patient and statements by the inventor of the mechanical heart, renowned cardiac surgeon Alain Carpentier MD, that the device had stopped abruptly.
One of the surgeons who participated in the implantation procedure, Daniel Duveau MD, said that the heart did not stop brutally. ‘During two hours, each time the device stopped, the system did everything it could to restart the pump. Despite a possible dysfunction, the system intelligently demonstrated its capabilities,’ he said, comparing the action to that of a doctor performing a cardiac massage.
The first patient, Claude Dany, 76, lived 74 days, which, noted lead surgeon Christian Latremouille MD, from the Hôpital Georges-Pompidou in Paris, widely exceeded the end point for the safety study. After a four-month review of the device and the causes of death of the first patient, French authorities approved the continuation of the clinical trial for safety and feasibility. In its press release, Carmat reported that two independent control committees monitoring the trial had met on 4 September 2014 and issued a report approving a continuation of the trial for the final two patients.
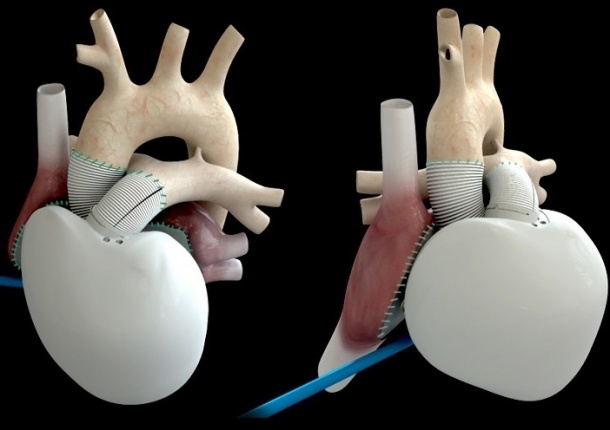
The Carmat artificial heart is the first device to completely replace a human heart and is fully contained within the thorax requiring no external pumps. Only two wires exit the body at the abdomen, one to supply power and the second to monitor device performance.
It is also the first artificial heart capable of adapting the blood supply according to a patient’s activity, varying from three to nine litres per minute, rather than having a constant Carmat repeated in its press release that, in conformance with good clinical practices, there would be no reporting of results of any of the implantations until the end of this safety and feasibility trial, unless required by ‘particular circumstances’.
08.12.2014





