Image source: Unsplash/Bill Oxford
News • COVID-19 diagnostics
Corona 'pool testing' increases worldwide capacities many times over
Researchers at the German Red Cross Blood Donor Service in Frankfurt headed by Professor Erhard Seifried, and the Institute for Medical Virology at the University Hospital Frankfurt at Goethe University headed by Professor Sandra Ciesek succeeded in developing a procedure that makes it possible to immediately and dramatically increase worldwide testing capacities for detecting SARS-CoV-2.
“This makes it possible for the implementation of expanded testing in larger population groups as demanded by all scientists and also politicians to be carried out sooner than previously thought, even in view of limited test kit resources,” says Seifried. The background of this news are laboratory investigations in which swab samples from mucous membranes of the throat or nose are combined using specified procedures in a buffer solution, and subsequently tested using what is known as the PCR procedure (polymerase chain reaction procedure, direct genome detection of SARS CoV-2). In the case of a negative result, all included samples have a reliable negative result. The pool testing has no influence on the detection limit. In the case of a positive mini-pool result, individual testing is carried out in previously reserved samples. The positive sample can then be identified within 4 hours.
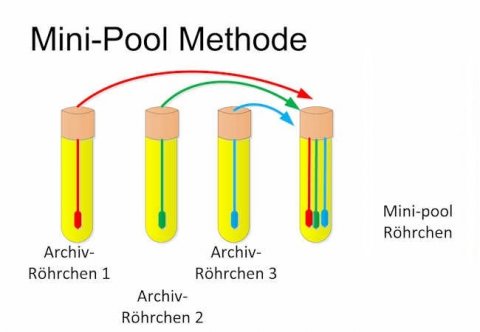
Graphics: Michael Schmidt, German Red Cross Blood Donor Service, Frankfurt Institute
The figure on the left shows the new mini-pool method. The swab is first placed in a reserve test tube and subsequently in a pool container. Since this pool method does not increase the volume in the pool container, no dilution and therefore no decrease in sensitivity is observed. Using independent ring trial samples (pre-test of the planned ring trial), that were provided by a ring trial organisation (INSTAND) authorised by the by the German Medical Association, it was able to be shown that the new mini-pool method can obtain laboratory results of equal quality as those obtained with individual testing. The new method was additionally investigated in a small field study on 50 unselected patient samples. The patient samples were pooled in 10 mini-pools of 5 samples each, and also tested individually in parallel. Of the 50 patient samples, 5 samples were SARS CoV-2 positive. These samples were distributed across 4 pools. All four mini-pools resulted in a positive PCR result. Mini-pools containing only samples from patients without SARS CoV-2 always resulted in a negative result.
Image source: Goethe University Frankfurt
With the new method, Germany can take the global fight against SARS-CoV-2 to a new a new level
Erhard Seifried
“On the basis of these laboratory results, in particular large cohorts and testing on asymptomatic individuals can be carried out, allowing a massive savings of test kits in particular,” says Ciesek. The research group is currently analysing options for further enlarging the pools. “The results give cause for optimism that when globally established, the procedure will quickly enable better information about the number of people actually infected,” explains Professor Schmidt from the Blood Donor Service. This allows the current number of approximately 40,000 tests per day in all of Germany to be immediately increased to 200,000 to 400,000 tests without reducing the high quality of the diagnosis. To continue efficiently implementing the strategy of early detection and isolation, an extension of screening tests is essential, especially for system-relevant professions such as doctors, nurses, police, firefighters, nursing homes, administration and the food industry. The rights to the invention, for which a patent is pending in Europe and the USA, is held jointly by Goethe University and the German Red Cross. The technology can be made available immediately to other interested institutions through Goethe University’s knowledge transfer company Innovectis.
Goethe University President Professor Birgitta Wolff describes the new analysis method as a “milestone”. “The more people who can be reliably tested for SARS-CoV-2, the faster the pandemic can be curbed.”
“With the new method, Germany can take the global fight against SARS-CoV-2 to a new a new level,” observes Seifried with optimism. The medical director of the University Hospital Frankfurt, Professor Graf, and the president of Goethe University both stressed the successful and trustful collaboration between the Blood Donor Service, the University Hospital, and Goethe University.
Source: Goethe University Frankfurt
01.04.2020