Breast tomosynthesis
A promising mammography screening technology
By Andrew Smith PhD, principal scientist at Hologic, Inc. in Bedford, Mass, is involved in research and development of digital imaging systems.
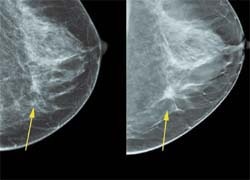
Mammography is an effective imaging tool to detect early breast cancer, and is the only screening modality proven to reduce mortality from breast cancer. Mammography is a very technically demanding radiographic procedure because it simultaneously requires high spatial resolution and good dose performance. High resolution is needed because some objects that must be depicted are very small microcalcifications, which can be visualised when they are as small as 200 microns. Dose performance is a requirement, because mammography is a screening modality and patient X-ray dose must be kept as low as reasonably acceptable.
The presence of overlapping tissue poses a significant obstacle in interpretation with conventional screening mammography. When screening mammography demonstrates questionable findings, follow-up diagnostic mammograms and other tests, such as ultrasound or MRI, or biopsy, ultimately determine whether the finding is significant. This process creates an anxiety for patients and induces additional healthcare costs for findings that frequently are found to be benign.
As evidence accumulates from clinical trials in the United States and Europe, breast tomosynthesis is on track to provide a superior alternative to the analogue and digital screenings available today. The industry has been talking about tomosynthesis for years. Now, finally, commercial systems are expected to be available soon.
Efforts to use tomosynthesis techniques for breast imaging were pioneered at Massachusetts General Hospital in the U.S. in the mid-1990s before the first digital mammography systems were commercialised. The early trials were promising, but limitations were apparent. Advances in image receptors, computer processing power, and digital mammography system designs have now made the application of breast tomosynthesis practical.
The principle of tomosynthesis is simple. During tomosynthesis acquisition, the breast is held stationary, and images are acquired at a number of different X-ray source angles. The dose of each image is kept low so that the total dose of all the images is similar to that of a conventional single breast mammogram. These images, known as projections, are then converted into 3-D cross-sectional slices through mathematical algorithms similar to CT reconstruction methods. The resultant 3-D image sets can be viewed on a computer workstation. These images are superior to digital mammograms because of the reduction of noise from breast tissue at different heights in the breast.
Tomosynthesis systems look much like digital mammography machines, and the breast is compressed in a standard way. Similarly to conventional mammography, the breast is imaged in the medio-lateral oblique (MLO) or the cranio-caudal (CC) orientation, although the tomosynthesis system should support the ability to acquire images in any desired view.
A high quality digital detector with rapid readout and minimal image distortion is important for breast tomosynthesis. Current digital mammography technology fulfils this requirement. Detector technology can be cesium iodide crystals on an amorphous silicon thin film transistor (TFT) array, or selenium on silicon TFT arrays. Selenium is an especially good material due to its high dose efficiency, i.e., its greater than 95% X-ray absorption at mammographic energies.
Another consideration in the design of tomosynthesis systems is the motion of the X-ray source during acquisition. The X-ray tube can move in a continuous or step-and-shoot motion. If the tube rotates continuously, short X-ray pulses are used to avoid blurring the image due to focal spot motion during each exposure. If step-and-shoot motion is employed, the gantry must come to a complete stop at each angular location before turning on the X-rays, otherwise vibration will blur the image. With continuous motion, scan speed must be slow enough, or each X-ray exposure short enough, to avoid image blurring due to focal spot motion. The angular range and number of exposures acquired during the scan are additional variables that need to be optimised. Minimising the total scan time is important, because patient motion will degrade the visibility of small objects in the breast.
In general, more exposures will allow reconstructions with fewer artifacts. This must be balanced against the fact that for a given total examination dose, more exposures will mean smaller signals for each of the individual shots. For sufficiently small exposures, imager receptor noise will dominate the image and degrade reconstructed image quality. More exposures can also increase the scan time, which degrades the image through patient motion.
In regards to angular range, a larger angular range gives superior reconstructed slice separation, where smaller angular ranges keep more structures in focus in a given slice. Increased separation might be desired for resolving two closely lying structures, but could impair the appreciation of a cluster of microcalcifications by having individual calcifications appear in different slices. A large lesion might have some of its spicules appear less sharp in any given slice if those spicules are far from the displayed slice. A larger angular range can also be a disadvantage if it requires a longer scan time.
Some commercial units like Hologic’s Selenia Dimensions now working its way through the FDA approval process, have dual functionality to perform both 2-D digital mammogram and breast tomosynthesis with the same unit. Therefore, it has all advantages of 2-D digital mammography, and in addition the ability to perform 3-D imaging. While the exact performance of 3-D imaging is still under investigation, it is likely that 2-D imaging will be a required operation mode for some time, such as to support magnification imaging.
Potential clinical benefits
Tomosynthesis should resolve many of the tissue overlap reading problems that are a major source of recalls and additional imaging in 2-D mammography exams. The biopsy rate should also decrease through improved visualisation of suspect objects. Some pathologies that are mammographically occult will be discernable through the elimination of structure noise. Finally, tomosynthesis may allow for improved detection of cancers in women with denser breasts that are currently not well served by 2-D mammography.
Tissue localisation
Because the location of a lesion in a tomosynthesis slice completely determines its true 3-D coordinate within the breast, needle localisation and biopsy tissue sampling methods can easily be done using the tomosynthesis-generated coordinates.
Faster review time
As the images are presented with reduced tissue overlap and structure noise, objects are expected to be visualised with improved clarity. This will likely lead to faster case review and more confident readings. Computer-aided detection (CAD) algorithms might also have improved accuracy.
Reduced compression pressure
In conventional mammography, breasts are highly compressed so as to reduce tissue overlap. High compression pressure is not needed for tomosynthesis imaging. Just enough breast compression to pull tissues out of the chest wall and keep motion at a minimum is adequate. Therefore, there is the possibility of less painful compression using tomosynthesis. If reduced breast compression is used, the X-ray energies may need to be raised so as to penetrate the thicker breasts more efficiently. In this case, it is important that the image receptor maintain its high quantum efficiency at the higher energies.
Contrast-enhanced imaging
Researchers have studied mammography using IV administered iodinated contrast agents. Using either dual energy or pre- and post-contrast imaging, they have observed enhancement of otherwise occult cancers and differentiation of benign from malignant tumours. While this research is still in its infancy, contrast- enhanced tomosynthesis images might allow for even greater malignant tumour to background contrast and visibility over that observed with 2-D contrast imaging, and could conceivably supplant MRI gadolinium breast imaging.
29.02.2008