A safe pacemaker for MRI scans
Report: John Brosky
‘There are few reasons to deny a patient an MRI scan, and nearly all of them are having a pacemaker,’ said Pierre Bordachar MD at the Centre Hopitalière Universitaire (CHU), Bordeaux, France. Yet one-in-five pacemaker patients will require an MRI scan within the first year of receiving a pacemaker, while more than half of all pacemaker patients will need such a scan at some later point in life after receiving the device, according to Jean-Nicolas Dacher MD at the celebrated cardiac centre in the CHU in Rouen.

Around two million Europeans have implanted pacemakers. Risks for patients with an implantable cardioverter-defibrillator (ICD) are slightly lower for MRI scans.
The MRI scan is ‘unmatched, irreplaceable and the indispensable reference for the diagnosis of cancers, neurological disorders, and increasingly, cardiac conditions,’ Dr Dacher said, adding that often computed tomography (CT) scans or ultrasound are often ‘ not a suitable alternative to MRI for many conditions.’
Exceptions are made, the two experts acknowledged during a presentation at this year’s annual congress of the French Cardiology Society, citing an especially strong pressure by neurologists for the MRI scans.
Dr Bordachar admits that, despite the discouragement in practice guidelines, MRI scans are conducted on as many as 12 patients per year at CHU-Bordeaux under careful protocols.
These protocols include reprogramming the MRI scanner, careful patient monitoring by a cardiologist during the scan and a thorough follow-up over several months because the three magnetic fields of the MRI can provoke diverse effects including arrhythmia, device damage, interference with the pacing function and an electronic reset of the device.
The immediate concern is the super-heating of lead wires on the device, and the follow up is required because force and torque exerted by the whipsaw effects of magnetic cross currents can shake or dislodge an implanted device.
Dr Bordachar said even after following careful and time-consuming protocols, Dr Bordacher said, ‘there are always going to be unpredictable complications.’
The only study of deaths from MRI have been by Werner Irnich MD, at the University Hospital Giessen, Germany, who compiled reports from 30 German medical centres and found that, from 1992 through 2001, six deaths resulted when pacemaker patients underwent an MRI scan.
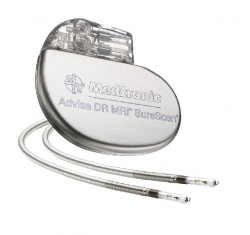
In this clinical context, the announcement at the French congress that the innovative Advisa pacemaker from Medtronic will be introduced in 2010 in Europe was welcomed by cardiologists.
The world’s first MRI-compatible pacemaker, the Advisa is re-engineered with reduced ferrous components to minimise the level of energy transmitted through the lead wire to the device connection point, a potential source for over-heating under magnetic forces.
The key feature for Medtronic’s novel pacemaker, to be introduced in 2011 in the USA under the name Revo, is the SureScan programming enabled during an MRI scan to eliminate the impact of electrical noise, such as disruptions to the pacing therapy. The SureScan feature temporarily suspends data collection and monitoring functions while allowing the device to continue providing asynchronous pacing, if needed.
Frédéric Anselme MD, from CHU-Rouen presented the preliminary findings from a Medtronic-sponsored EnRhythm MRI SureScan clinical trial to assess safety and effectiveness of the new device with 470 patients from 53 centres in Europe and the USA.
To date, 211 patients with the new pacemaker underwent head and lumbar scans with 1.5 tesla scanner while the remaining patients served as a control group.
‘The big news is that patients have been 100% complication-free,’ Dr Anselme reported, saying there were incidents of ventricular arrhythmias or asystole, no differences in pacing and sensing, nor evidence of either clinical or device complications.
‘This data is promising as we have observed no lead-performance issues or unexpected MRI effects in this patient group to date,’ said Professor Torsten Sommer, chief of the cardiovascular section of the radiology department, University of Bonn, Germany, and European lead radiology investigator for the clinical trial. ‘MRI is unmatched and irreplaceable in the diagnosis of cancer and neurological disorders. As the necessity for MRI grows, it is critical to introduce a solution that allows pacemaker patients safe access to MRI.’
The Advisa pacemaker will be available for implantation in patients as early as June this year, according to Christèle Pelade, Manager for Implanted Diagnostic & Monitoring Devices with Medtronic France.
14.05.2010