Article • Electrospinning
Renewing the promise of bioabsorbable implants
Electrospun materials bring a spark of hope to a cardiovascular landscape darkened by setbacks for reabsorbable stents.
Report: John Brosky
It was famously said that implanting a device in a person to cure a disease is to implant a new disease. Simply put, the human body will continually fight against foreign materials, leading to chronic inflammations or repeated interventions. Which explains the recent excitement among cardiologists for a new generation of materials that can repair a diseased condition, and then disappear as the body absorbs the foreign material.
The earliest advances in this field were made with bio-reabsorbable stents (BRS), which have also delivered the greatest setbacks. Two- and three-year results for the leading stent, the Absorb BRS from Abbott Vascular, not only failed to demonstrate the promised benefits, but also in some trials showed a dangerous tendency for in-stent thrombosis. In the August 2017 issue of EuroIntervention, Editor-in-Chief Patrick Serruys noted the long-term follow-up data from randomised trials ‘sometimes contradict themselves’, and show that, ‘we went from the best result to the worst result.’ With improved technology, he concluded, ‘We will reach the promised land, which is not for tomorrow but for the day after.’
Beyond stents, which utilise a hardened bioabsorbable polymer, other cardiovascular applications for bioabsorbable materials have shown more promising long-term results with the first in-human trials.
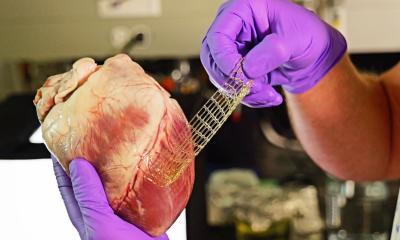
The technology that may open a new path to the Promised Land is electrospinning, where an electrospray technique combined with the spinning of fibres creates a highly versatile soft fabric for implantation devices that the body can absorb over time.
The medical need has been better defined, which has created a greater demand, and as a result, there is a market today
Benoit Studlé
While this technology has been known for 100 years, and for decades has been used to produce absorbable sutures for surgical closings, it is only recently that new devices using the materials have emerged. According to Benoit Studlé, the CEO of Statice, which designs and manufactures these next-generation cardiac implants, the rediscovery of this technology is due to a progressive evolution in the savoir-faire for engineering unique chemical and mechanical properties. ‘The medical need has been better defined, which has created a greater demand, and as a result, there is a market today. This is a great motivator,’ he said, adding that there are multiple projects moving in the cardiology pipeline.
As a contract manufacturer, Studlé can not speak about projects being developed by innovative physicians and start-up entrepreneurs at Statice – except to say the company is a partner in a pan-European program TEH-TUBE, [tissue engineering of the right heart outflow tract by a biofunctionalised bioresorbable polymeric valved tube] and REVAMED, a €4 million program to develop implantable biosynthetic patches for drug delivery to aid with surgical closings, wound dressings and pain management. Statice engineers are also creating electrospun fabrics for an aortic valve, but this is still in an early stage of development.
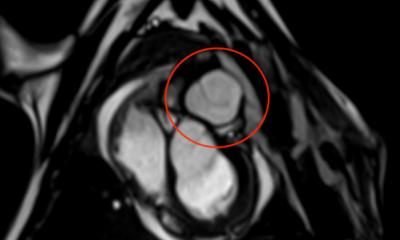
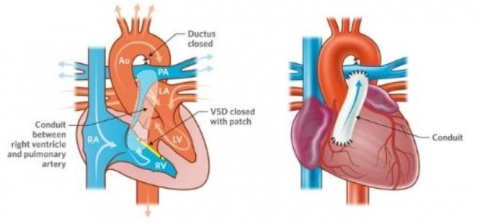
A Swiss firm that can speak publicly about its work in cardiovascular applications is Xeltis, which, during EuroPCR 2017, renewed the hopes for bioabsorbable implants in a crowded scientific session. Xeltis presented the 24-month results for a pulmonary heart valve to correct or reconstruct right ventricular outflow tract in 10 children, as well as the first study results from the company’s preclinical aortic valve program.
The prestigious panel of key opinion leaders would have been enough to pack the room, as it included presentations by Serruys, who is a professor of Cardiology Imperial College London; Thierry Carrel, from the University Hospital Bern Clinic, who also serves on the editorial board of several international journals; the internationally renowned cardiovascular pathologist Renu Virmani; and, Martin Leon, the director at the Center for Interventional Vascular Therapy at Columbia University Medical Center and New York-Presbyterian Hospital.
Elazer Edelman, who directs the Harvard-MIT Biomedical Engineering Center and is a member of the U.S. FDA’s Science Board, chaired the session. The Chief Technology Officer and co-founder of Xeltis, Martijn Cox, explained the principle of what his company calls endogenous tissue restoration using electrospun fabrics.
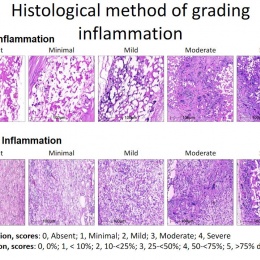
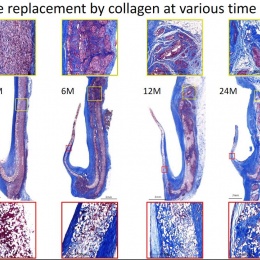
The human body colonises implanted scaffolding by creating proteins and collagen to build new tissue, while macrophages also attach to the structure and simultaneously dissolve the foreign material. Serruys suggested the dynamic tension between the rate of destruction and the rate of construction would be the subject of debate for a long time. ‘How sharp, how long, how fast? There may yet be surprises, we may discover new enemies,’ he said.
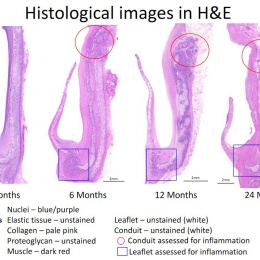
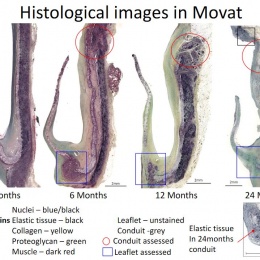
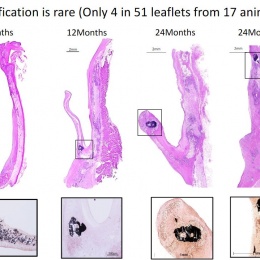
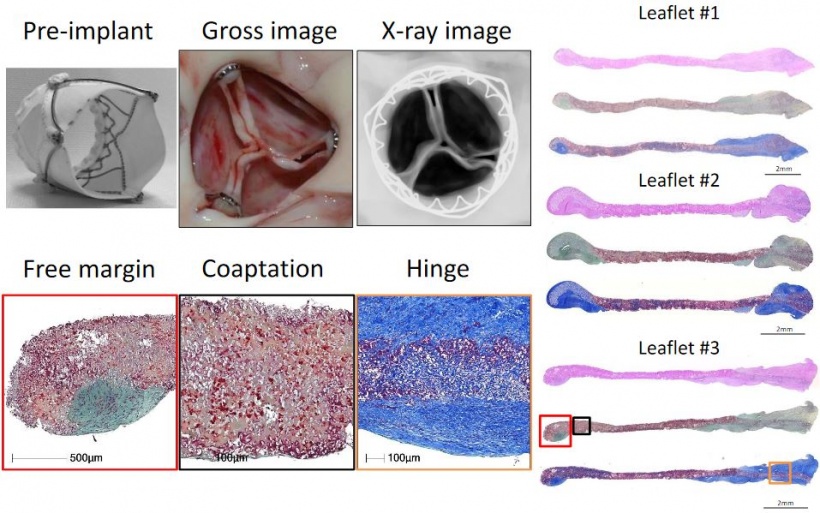
Virmani showed that among the children implanted with the Xeltis valved conduit, the diameter of the valve is not only well-maintained after two years, it is enlarging, ‘so that as the child will grow, this conduit will allow enlargement of the pulmonary trunk as well as the valve’.
Yet, a conduit is not an aortic valve, she said, showing histological evidence to demonstrate a critical inconsistency in the creation of new tissue at the hinge point of the aortic valve. ‘We need to think completely differently about how colonisation and tissue formation is achieved,’ she said. ‘Unfortunately, we cannot learn this on the bench top The promised land of synthetic implants that can heal is clearly not for tomorrow.

Yet, as Edelman noted, there is urgency in cardiovascular interventions. ‘Today heart valves are made from the hide of a very small herd of animals in a very small part of the world. This forces us to consider where these materials are going to come from, as the demand grows greater,’ Edelman pointed out.
According to Leon, in a summary statement: ‘This innovative treatment approach has the potential to reduce complications, re-interventions and healthcare costs, while improving quality of life for patients with heart valve disease. This would represent a major leap forward in heart valve therapy.’
30.08.2017