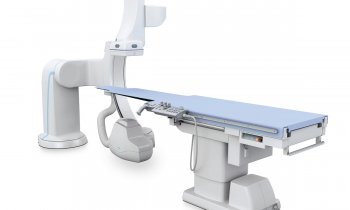
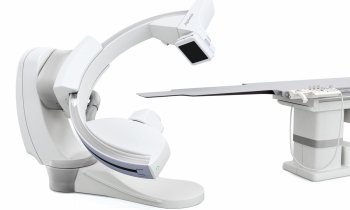
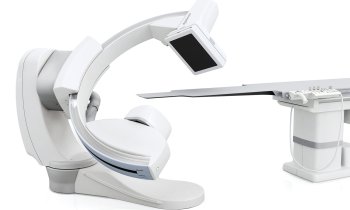
Renal denervation moves beyond the HTN-3 disaster
Clinicians and companies remain committed to a procedure that failed to demonstrate efficacy against a sham-control group in pivotal clinical trial
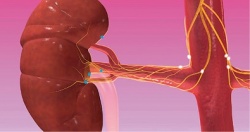
One year ago the enthusiasm for treating resistant hypertension with renal denervation was festive. ‘That party is over,’ laughed Mano Iyer, founder and COO for ReCor Medical, one of the dozen companies offering novel devices to ablate nerves in the renal artery. ‘Last year everyone was riding on the coat tails of Medtronic,’ he said. ‘Now the playing field has been levelled and we all have an opportunity to differentiate with what we believe is a superior technology.’
The transforming event that trashed the party came in January this year when Medtronic reported that its SYMPLICITY-HTN 3 pivotal trial of renal denervation in the United States failed to meet its primary endpoint for efficacy against a sham-control group of patients.
In March 2014, at the meeting of the American College of Cardiologists, the trial investigators revealed details that confirmed Symplicity HTN-3 was indeed the most rigorous renal denervation clinical trial conducted to date, and that the validated data did indeed show the therapy was no more effective than compliance to medications to lower blood pressure.
The news recalled for many interventional cardiologists the Black Tuesday in Barcelona in 2006 when the then-emerging practice of implanting stents for coronary revascularization failed to match the efficacy of coronary artery bypass graft surgery (CABG) in the landmark SYNTAX trial.
Despite that setback, stenting of arteries has grown to become a standard of care for revascularisation. Both clinicians and companies invested in the development of renal denervation expect that the procedure will similarly overcome the disappointment of the Symplicity trial to find its place as a therapy.
The field of renal denervation is ‘too interesting and too young to be written off,’ stated Felix Mahfoud MD, an interventional cardiologists at the Saarland University Hospital (Homburg, Germany) who has consulted with Medtronic, St. Jude Medical (St. Paul, Minnesota), and Recor.
Speaking as a course chairmen for the newly formed Resistant Hypertension Course, he said in a statement at EuroPCR, ‘We see the results of this trial [Symplicity-HTN 3] as neutral and after a careful assessment of this study have identified various potential procedural and methodological considerations that could partly account for the study’s results.’
The General Manager for Renal Denervation (RDN) at Medtronic, Nina Goodheart stated in an email, ‘As the leader in RDN Medtronic will continue to support our global HTN [hypertension] clinical program to better understand the potential of RDN in uncontrolled HTN. ‘
‘We are continuing our analyses of Symplicity HTN-3 and are committed to better understanding the confounding factors observed in this trial. We believe there are many factors that may have contributed to the observed efficacy results in Symplicity HTN-3, including key variables that have arisen such as population differences and medication and procedural variability in Symplicity HTN-3 versus other Symplicity studies,’ she stated.
St. Jude Medical is just as heavily invested in renal denervation as its rival Medtronic, and through the vice president for corporate relations, Rachel Ellingson, said that it is also ‘committed to this space’.
‘True innovation takes time and persistence to develop,’ she said. ‘The good news is that industry, academics and regulators are interested in talking about how to develop evidence that is supportive of the therapy, and whether new trial designs might help bring this therapy to patients with severe high blood pressure.’
The parade of trial results for a variety of new devices continues from Boston Scientific, Terumo and the Cordis unit of Johnson & Johnson. While the causes of Symplicity-HTN 3 failure to prove efficacy may be confounding, one clear result of that study, according to Mahfoud, is that all clinical trial in the field moving forward will require a control arm.
‘Sham-control arms will be a real problem in Europe,’ he said, declining to elaborate.
Separately, Iyer with ReCor, who is based in Amsterdam, explained. ‘If you talk to European physicians they will tell you that a sham-control may be scientifically important but not clinically relevant and that practically it is very difficult,’ he told European Hospital. ‘They are not believers here in Europe that a sham is necessary. They will ask how they can ethically prescribe a sham procedure if there is a procedure for a patient coming in who is suffering, with no alternatives. They will tell you this patient is going to come back with a significant cardiovascular event if we don’t get their blood pressure down. Meanwhile here is a technique that is proven to be safe that might help.’
Roland Schmieder MD, a nephrologist specialising in hypertension at the University of Erlangen in Germany, told European Hospital that ‘Moving forward with renal denervation means two things. First we need to move forward with more robust study designs. It does not need to have sham-control, but it does need to be randomized with a real control group.’
‘As for the technology, we have heard of new technologies for a more reliable delivery of the energy, such as ultrasound, or 360-degree radiofrequency. What will become important are technologies for making the procedure less operator-dependent with reproducible effects. We are not there yet.’
30.08.2014