Image credit: Professor Raimondo Ascione, University of Bristol
News • SEBS material safety
New artificial heart valve passes long-term test in animals
The next generation of an artificial heart valve made from a new plastic material could be a step closer to bedside. The research, led by the Universities of Bristol and Cambridge, demonstrated that the polymer material used to make the new artificial heart valve is safe following implant in an animal model for six months.
The study, funded by the British Heart Foundation (BHF) is published in the European Journal of Cardio-Thoracic Surgery.
Currently, patients who need heart valve replacements face trade-offs. Mechanical heart valves are durable but require lifelong blood thinners, while biological (animal tissue) heart valves are more natural but tend to wear out more quickly. The new kind of artificial heart valve made from a special plastic called SEBS (styrene-block-ethylene/butyleneblock-styrene) might offer the best of both worlds — but the safety of these new material must be confirmed before they can be tested in humans.
Our findings could mark the beginning of a new era in artificial heart valve being made from the new plastic material — one that may offer safer, more durable and more patient-friendly options for patients of all ages, with fewer compromises
Raimondo Ascione
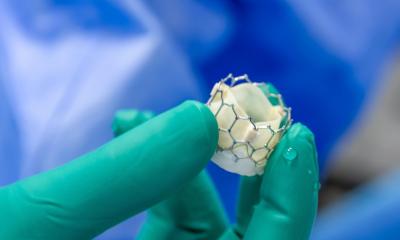
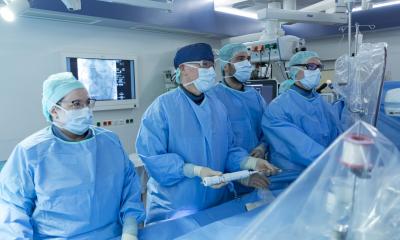
In this new study, researchers tested a research-grade prototype of a new heart valve made entirely from SEBS polymers in a preclinical cardiac surgery model that mimicked how these valves might perform in humans. The team implanted the prototype polymeric artificial heart valve in seven sheep, which was carried out by an independent preclinical facility in Paris, France. The animals, and their wellbeing, were then monitored over six months, to examine potential long-term safety issues associated with the plastic material such as mineral build up (calcification), material dissolving, blood clots, damage of the heart tissue in contact with the material, cancer, cell death, liver or kidney failure, nutrition and weight and other issues as determined by the regulator.
The research found after six months there was no evidence of harmful calcification (mineral buildup) or material deterioration; blood clotting or signs of cell toxicity. Animal health, wellbeing, blood test results and weight gain overtime were all stable and normal, and the prototype heart valve functioned well throughout the testing period, with no need for blood thinners.
Raimondo Ascione, NHS Adult Cardiac Surgeon, Professor of Cardiac Surgery and Translational Research and Director of the Translational Biomedical Research Centre (TBRC) at the University of Bristol, clinical lead on the project and corresponding author, said: “Currently 1.5 million patients around the world require heart valve replacements every year. More than 35 million patients’ heart valves are permanently damaged by rheumatic fever and with an ageing population this figure is predicted to increase four to five times by 2050. Our findings could mark the beginning of a new era in artificial heart valve being made from the new plastic material — one that may offer safer, more durable and more patient-friendly options for patients of all ages, with fewer compromises.”
Geoff Moggridge, Professor of Chemical Engineering Science at the university of Cambridge and biomaterial lead on the project said: “We are pleased that the new plastic material has been shown to be safe after 6 months of testing in-vivo. Confirming the safety of the material has been an essential and reassuring step for us, and a green light to progress the new heart valve replacement toward bedside testing.”
The researchers early bench results suggest that their artificial heart valves made from SEBS could feature excellent durability and hemo-compatible i.e. without the need for lifelong blood thinners.
While this is still early-stage research and limitations of the small sample size, the findings are a major hurdle toward future human testing. The next step will be to develop a clinical-grade version of the SEBS polymer heart valve and test it in a larger preclinical trial before seeking approval for a pilot human clinical trial.
Source: University of Bristol
21.08.2025