itm Rhenium-PTA/PTCA®
'An effective, safe treatment for prophylaxis of restenosis in periphery and cardiology' — new generation of Endovascular Brachytherapy gains full CE-certification. Constricted blood vessels (stenosis and restenosis) can now sustainably be treated and kept open through the local treatment with beta radiation.
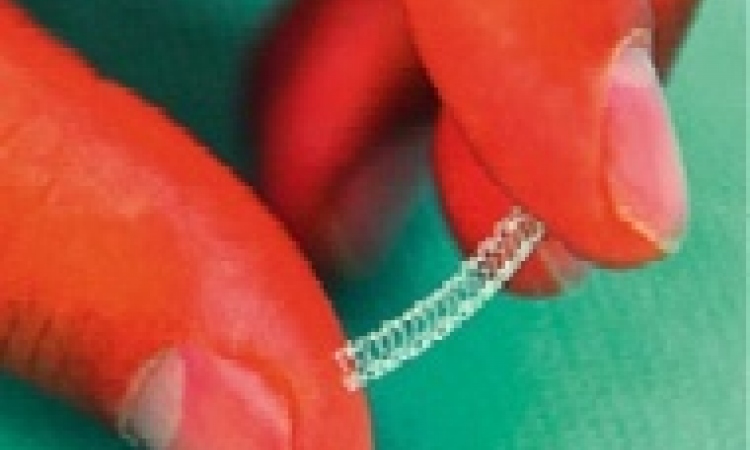
itm FlowMedical GmbH reports that its itm Rhenium-PTA/PTCA® now provides a proven and effective solution for the treatment of stenosis and re-stenosis, especially in peripheral blood vessels, where other treatment alternatives have no real significance. This advantage could, among other factors, be established through the significantly improved performance of the beta emitter Rhenium-188 and the ASK-Application System.’
Although bare-metal and drug-eluting stents are commonly used in coronary treatments (PTCA), the occurrence of in-stent-restenosis still poses a severe and prevalent problem, the manufacturer points out. ‘In the periphery, where medical tools like stents still have little or no relevance, restenosis is an even more common complication of PTA treatments. Peripheral arterial disease mainly affects patients suffering from diabetes. Main consequences are myocardial infarctions in cardiology as well as organ failures or amputations in periphery.’
For many years Endovascular Brachytherapy (EVBT) has been considered an effective treatment for the prophylaxis and treatment of stenosis and restenosis. ‘However, many of the traditional EVBT treatments, primarily applied in cardiology, shared some fundamental common shortcomings. With the itm Rhenium-PTA/PTCA® a new generation of brachytherapy is now available that systematically resolves issues experienced in the past – and is applicable and approved not only for cardiology but also for the treatment of peripheral arterial diseases.’
Due to its highly efficient treatment characteristics and outstanding safety standards, the firm adds, the itm Rhenium-PTA/PTCA® offers the solution to the shortcomings of conventional EVBT treatments. Its advantages include:
• Simple application in the angio suite – no relocation of the patients required
• Flexible treatment of vessels geometries, also with large diameters and long lesions
• Efficient process through high activity concentration and short treatment time
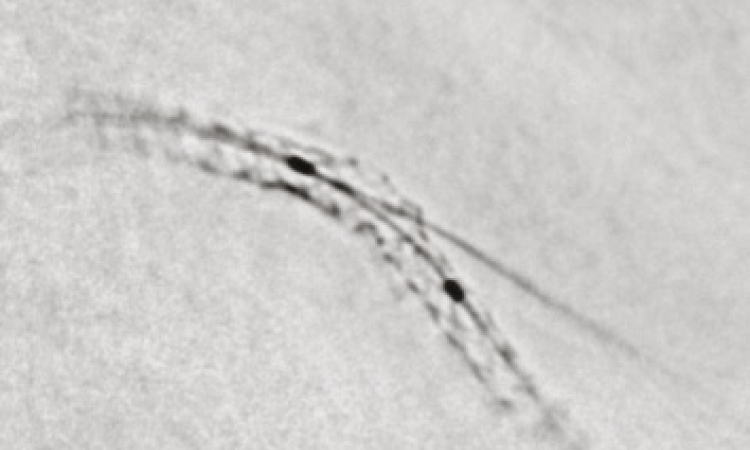
• Safe and homogeneous irradiation through the systems self-centring characteristics
• Optimised radiation protection through a specially developed application system (ASK)
• Just-in-time delivery and availability of Rhenium-188 at short notice
• Simple and economical waste management due to short half-life (17h) of the Rhenium-188
itm Flow Medical GmbH also reports that – with the help of a specially developed generator – it is delivering the liquid Rhenium-188 ready-to-use in the required activity concentration and volume – without any necessity for additional concentration or preparation. ‘Therefore, the ideal dose can be applied in a short overall treatment time of 3 to 7 minutes. Being a beta-emitter Rhenium-188 has a very short path length and shows fast dose reduction in tissue which guarantees that the radiation penetrates only a few millimeters into surrounding tissue.
Therefore, only the immediate vessel wall is irradiated with practically no radiation of surrounding tissue. Furthermore, the catheter’s self-centering characteristics ensure that the vessel walls are irradiated homogenously, making the treatment more medically efficient.
When the treatment is complete, the short half life of Rhenium-188 of only 17 hours ensures that residual radiation levels in used materials drop very quickly which significantly simplifies otherwise complicated waste management.
The method also provides an optimum standard in radiation protection. ‘Due to the specially developed application system (ASK), interventions can be performed at extremely low levels of radiation exposure to personnel and patients. Independent measurements of the German Federal Agency for Radiation Protection (BfS) have proven the ASK’s excellent protective features making it appropriate for widespread use in routine clinical application.’
This September, the itm Rhenium-PTA/PTCA®, which is distributed by itm FlowMedical GmbH, received full CE-certification for application in cardiology and the periphery. The device comprises the specially developed Application System (ASK), liquid beta emitter Rhenium-188, specially certified PTA and PTCA-catheters as well as several accessories, and is currently being introduced for routine applications in cardiology and periphery. The treatment can be reimbursed by public and private insurers in Germany, the manufacturer points out.
From 2004, Augsburg Central Hospital has successfully used the treatment and achieved excellent clinical results, the company adds. ‘16 months after treatment the restenosis rate is at only 13.6% – even when treating multi-morbid patients. (Conventional treatments, such as the drug eluting stent or drug-coated balloons, show a restenosis rate of 20% or higher within six months after an intervention).
itm FlowMedical
itm FlowMedical GmbH became a spin off of ITM AG in 2007, created to focus on the CE-certification, sales and marketing of the system. The company profits from the broad scientific and technical expertise of ITM AG and its collaborators in fields including medical engineering, radio chemistry, radiation protection, physics and radio pharmacy.
Production and development facilities are established and embedded in the industrial user centre (IAZ) in the direct vicinity of the new neutron-source FRM-II Heinz Maier-Leibnitzin Garching, near Munich.
The investments into the neutron source and, therefore, access to the scientific and industrial nuclear infrastructure on the campus generate a unique environment, which is of central importance to the development and commercial production of medical radioisotopes such as the Rhenium-188.
New devices that significantly improve radiation protection for example during the injection of radiopharmaceuticals in nuclear medicine are currently in development.
Contact details: itm FlowMedical GmbH, Lichtenbergstr. 1, 85748 Garching near Munich/ Germany.
Telephone: +49-89-28913940,
Email: info@flowmedical.com
www.flowmedical.com
28.10.2008