Sponsored • Pioneering cardiology
Implantable cardiac monitor gets diagnosis in just three days
It started as a fairly typical case: The 79-year-old patient had suffered unexplained dizziness for years. To diagnose why, the cardiology team at Sweden’s Kalmar Hospital performed echocardiograms, Holter ECGs and other tests. However, these tests showed normal sinus rhythm and thus were inconclusive.

Dr Hendrik Schreyer, Dr David Olsson and Professor Jörg Carlsson decided to use Biotronik’s recently CE-approved Biomonitor III home monitoring system to finally identified the problem – becoming the first team in the Nordic region to inject the new implantable cardiac monitor (ICM). Three days later, during the daily data transmission, the monitor detected an arrhythmia and the hospital soon received the relevant clinical and technical information. The physicians then informed their patient that they had found atrial fibrillation and an asystole with an eight-second pause. Based on the diagnosis, they recommended pacemaker implantation.
‘She was very pleased to get a call from us after only three days,’ said Schreyer, who performed the injection. ‘The device detected the problem very reliably. This is important because patients with ICMs often wait around four months until another episode occurs that gives us the information we need to make an accurate diagnosis.’
Recommended article
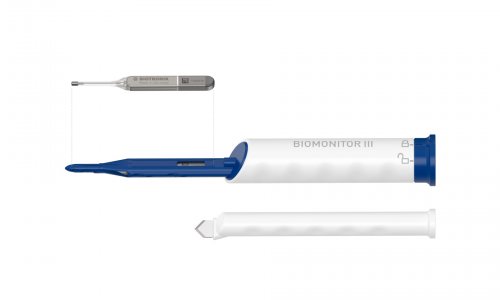
News • Biomonitor III
The next generation injectable cardiac monitor
Biotronik announces the market release of its new injectable cardiac monitor (ICM), Biomonitor III, following approval in the CE region. The novel device is designed to help patients with irregular heart rhythms by documenting suspected arrhythmia or unexplained syncope with increased clarity. As the most common type of arrhythmia, 33.5 million patients worldwide suffer from atrial fibrillation…
She was surprised, in the most positive way possible, that she could go home immediately afterwards and had no complaints at all
Hendrik Schreyer
The Biomonitor III is designed to monitor a patient’s heart rhythm long-term, transmitting data to the physician on a daily basis, to help them detect arrhythmia. Schreyer credits the system’s signal quality for helping to simplify the diagnosis, noting r-wave amplitudes as high as 2 mV being registered on ECG readouts from the device. ‘So far, I haven’t experienced another device with such a high signal quality. Most are under 1 [mV].’ He also notes the cardiac monitor’s clear p-wave visibility; a particularly important aspect for spotting atrial fibrillation.
The team finished the injection procedure in less than four minutes. Schreyer says the extra stability the device’s tools afford helps to ease the procedure, because they allow an implanting physician to steady the main tool with his or her entire hand.
The ease of the injection procedure was quickly apparent to the patient as well. ‘She was surprised, in the most positive way possible,’ Schreyer noted, ‘that she could go home immediately afterwards and had no complaints at all.’
Source: Biotronik
31.08.2019