Image source: Adobe Stock/LariBat
News • Neuroscience & Pediatrics
Gene therapy could relieve post-hemorrhagic hydrocephalus
Premature infants, especially very low birthweight babies, are at risk for intraventricular hemorrhage. A frequent complication of these brain bleeds is hydrocephalus, an accumulation of cerebrospinal fluid (CSF) in the brain ventricles that can gravely disrupt brain development. If hydrocephalus develops, a child may need shunt operations throughout life to manage the fluid buildup.
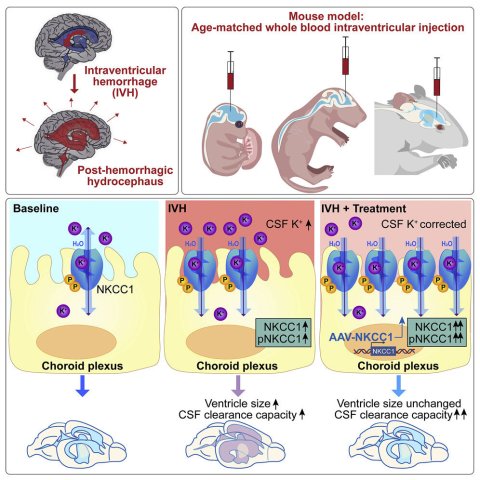
Could a better solution lie in the choroid plexus, the CSF-producing tissue within the ventricles? In a groundbreaking study in Neuron, led by Maria Lehtinen, Ph.D., at Boston Children's Hospital, targeting the choroid plexus with gene therapy accelerated clearance of the CSF in a mouse model of post-hemorrhagic hydrocephalus.
Study co-author Benjamin Warf, MD, a neurosurgeon in the Hydrocephalus Program at Boston Children's, is excited about the findings. "Tiny, pre-gestational age infants are at higher risk for complications from early shunt placement and are less likely to be successfully treated by endoscopic third ventriculostomy with choroid plexus cauterization (ETV/CPC)," he says. "The ability to avoid or delay operative intervention by manipulating the choroid plexus with gene therapy could be an enormous breakthrough in the care of these children."
Harnessing an ion transporter to clear fluid
How post-hemorrhagic hydrocephalus arises has not been clear because of the difficulty in studying fluid dynamics in the brain. Lehtinen, whose research focuses on the choroid plexus, the CSF, and brain development, decided to look at how bleeding into the brain's ventricles changes the chemistry of the cerebrospinal fluid.
"We thought, maybe we could try to understand how blood is interacting with the developing brain, particularly from the fluid perspective," she says. "Our question was, how does tissue that regulates CSF—the choroid plexus—respond to blood?"
Two fellows in her lab, Huixin Xu, Ph.D., and Cameron Sadegh, MD, Ph.D., teamed up to do the experiments. One of the first things they saw was that after a simulated brain bleed, potassium levels in the CSF were elevated—especially in newborn mice. They then observed that when blood was present, an ion transporter on the choroid plexus surface, NKCC1, became activated.
NKCC1 can move ions in two directions, either into or out of the CSF. In the presence of blood, the team found, NKCC1 moved potassium out of the CSF. The fluid went along with the ions—preventing ventricles from becoming enlarged.
"This is a self-rescuing mechanism," says Xu, "but when there's a severe bleed, it's not enough to reverse hydrocephalus in younger animals, which have low levels of NKCC1."
Translating the findings
What if NKCC1 could be boosted? Using an adenovirus as a gene therapy vector—one that naturally homes to the choroid plexus—Xu and Sadegh delivered additional NKCC1 to augment natural levels. In response, the mice were better able to clear CSF from their ventricles and had a reduction in ventricle size.
"Since not all brain bleeds lead to hydrocephalus, this work sheds light on how the choroid plexus might be helping some patients avoid the need for shunt surgery by acting quickly to restore ionic and fluid homeostasis," says Sadegh.
This likely represents the first gene therapy to treat any form of hydrocephalus and might lead to new treatment options beyond the usual surgical approaches
Maria Lehtinen
Lehtinen and her colleagues believe that improving fluid clearance could also help stabilize premature newborns with brain bleeds, tiding them over until they are old enough to have a shunt placed or undergo ETV/CPC.
"Some premature infants have progressive post-hemorrhagic hydrocephalus that demands treatment when they are still too tiny for either ETV/CPC or placement of a permanent shunt," elaborates Warf, who pioneered the ETV/CPC procedure.
"Surgeons often place a simple temporary shunt in these cases, but this poses a risk of serious infection. A non-operative way to manage hydrocephalus very early on could allow children to have ETV/CPC when they are older and thus help spare the majority from life-long shunt dependence. In addition, after temporary treatment, the hydrocephalus can sometimes resolve on its own once the blood clears, and then no further treatment is necessary."
Lehtinen and her colleagues want to try moving this gene therapy into a large animal model, and are in discussions with Warf about patients in whom the approach would be most appropriate.
"This likely represents the first gene therapy to treat any form of hydrocephalus and might lead to new treatment options beyond the usual surgical approaches," says Lehtinen.
Source: Children's Hospital Boston
25.04.2023