First pacemaker with event-triggered IEGM transmissions
New Biotronik pacemaker series offers daily automatic data transmission without relying on patient interaction and gain CE mark
Biotronik, a leading manufacturer of cardiovascular medical technology, announced CE approval for its new Eluna pacemaker series. The new generation of pacemakers includes single and dual-chamber as well as cardiac resynchronization (CRT-P) devices.
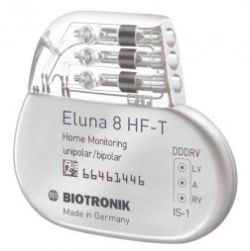
The first implantations are currently taking place worldwide, marking yet another step forward in quality for a company that has perfected the design and engineering of its products for over 50 years.
With unique event-triggered IEGM transmissions, the new BIOTRONIK pacemaker series innovates advanced patient monitoring and allows physicians to receive an IEGM along with a notification if atrial fibrillation (AF) occurs. By notifying physicians of clinical events quickly and reliably, patient therapy can be adapted at the earliest stage possible, preventing the worsening of a patient’s condition and the occurrence of secondary diseases. Since nearly 25 percent of pacemaker patients suffer from AF, a type of atrial tachyarrhythmia that can be difficult to detect due to its silent nature, continuous and reliable monitoring is crucial.1 BIOTRONIK Home Monitoringis proven to have the most reliable data transmission rates yet.2
“The new BIOTRONIK pacemaker series is truly a step forward in terms of reliability and patient security,” commented Hendrik Bonnemeier, MD, of the University Clinic Schleswig-Holstein, Kiel, Germany. “Its unique ability to transmit automatic, event-triggered IEGMs really helps me deliver the best therapy, letting me know immediately if something happens to the device or patient post-implantation. The sooner I know, the sooner I can react, and that may save my patient’s life.”
CRT-P Devices Offer Crucial Improvements in Heart Failure Treatment
Numerous studies have shown that devices with BIOTRONIK Home Monitoring aid patient therapy and significantly improve quality of life by reducing instances of hospitalization and stroke. In fact, in the
IN-TIME study, BIOTRONIK Home Monitoring was shown to reduce mortality in heart failure patients by more than 50 percent, making BIOTRONIK CRT devices especially important in effective heart failure therapy.3
Additionally, the new BIOTRONIK device family features ProMRI full-body scan in the single and dual-chamber pacemakers, and ProMRI capability in the CRT-P devices, enabling patients to undergo crucial MRI scans on nearly every part of the body.
“If the goal of a pacemaker is to help the patient live life to the fullest, almost to the point where he forgets he has a pacemaker, then the new BIOTRONIK pacemaker series truly succeeds,” commented Daniel Gras, MD, of the New Clinic Nantes, France. “It is also truly astonishing how far ProMRI technology has come in recent years. Now that there is no longer an exclusion zone, I can diagnose any kind of comorbidity.”
“With the development of our new pacemaker series, we have once again demonstrated our commitment to excellence for life,” commented Christoph Böhmer, President International at BIOTRONIK. “Now that remote monitoring and MR conditional devices are highly recommended in the ESC guidelines, we are proud to say that our new pacemaker series reflects reliable and innovative care that follows these guidelines, with a longevity that is truly the best on the market.”
First implanters of the new pacemaker series are:
Prof. Hendrik Bonnemeier, MD, University Clinic Schleswig-Holstein, Kiel, Germany;
Prof. Erik Friedrich, MD, St. Elisabeth Clinic, Saarlouis, Germany;
Daniel Gras, MD, New Clinic Nantes, France;
Prof. Amos Katz, MD, FESC, and Vladimir Khalameizer, MD, Barzilai Medical Center, Ashkelon, Israel.
For more information, visit: www.biotronik.com
References:
1 Funck et al., Europace (2014), 16(3): 354-362.
2 Varma et al., Europace (2011), 13(3).
3 IN-TIME study results were presented in Sept. 2013 at the European Society of Cardiology conference.
27.03.2014