News • XDR Klebsiella pneumoniae
Antibiotic resistance in Europe: Hospitals are part of the problem
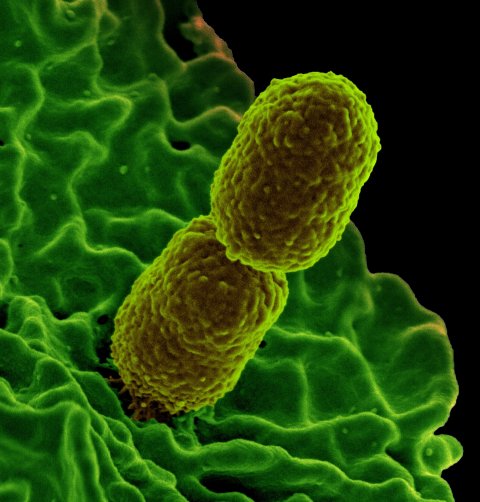
New research has found that antibiotic-resistant strains of Klebsiella pneumoniae, an opportunistic pathogen that can cause respiratory and bloodstream infections in humans, are spreading through hospitals in Europe.
Certain strains of K. pneumoniae are resistant to the carbapenem antibiotics that represent the last line of defence in treating infections and are therefore regarded as extremely drug resistant (XDR). During a Europe-wide survey of the Enterobacteriaceae family of bacteria, researchers at the Centre for Genomic Pathogen Surveillance, based at the Wellcome Sanger Institute, University of Freiburg and their partners, analysed the genomes of almost 2,000 K. pneumoniae samples taken from patients in 244 hospitals in 32 countries. The results, published in Nature Microbiology, will inform public health efforts to control the spread of these infections in hospitals across Europe.
Our findings imply hospitals are the key facilitator of transmission – over half of the samples carrying a carbapenemase gene were closely related to others collected from the same hospital
Sophia David
It is estimated that 341 deaths in Europe were caused by carbapenem-resistant K. pneumoniae in 2007; by 2015 the number of deaths had increased six-fold to 2,0941. The high number of deaths is down to the fact that once carbapenems are no longer effective against antibiotic-resistant bacteria, there are few other options left. Infants, the elderly and immuno-compromised individuals are particularly at risk.
The survey2 is the largest of its kind and is the first step towards consistent surveillance of carbapenem-resistant bacteria in Europe. More than 2,000 samples of K. pneumoniae were collected from patients across the 244 hospitals and sent to the Wellcome Sanger Institute, where the genomes of 1,700 of them were sequenced. Researchers identified a small number of genes that, when expressed, can cause resistance to carbapenem antibiotics. These genes produce enzymes called carbapenemases, which ‘chew up’ the antibiotics, rendering them useless. Of concern to public health is the recent emergence of a small number of ‘high-risk’ clones carrying one or more carbapenemase genes, which have spread rapidly. It is thought that the heavy use of antibiotics in hospitals favours the spread of these highly-resistant bacteria, which outcompete other strains that are more easily treatable with antibiotics.
Dr Sophia David, first author of the study, based at the Centre for Genomic Pathogen Surveillance, said: “The ‘One Health’3 approach to antibiotic resistance focuses on the spread of pathogens through humans, animals and the environment, including hospitals. But in the case of carbapenem-resistant Klebsiella pneumoniae, our findings imply hospitals are the key facilitator of transmission – over half of the samples carrying a carbapenemase gene were closely related to others collected from the same hospital, suggesting that the bacteria are spreading from person-to-person primarily within hospitals.”
Antibiotic-resistant bacteria samples were also much more likely to be closely related to samples from a different hospital in the same country rather than across countries – suggesting that national healthcare systems as a whole play an important role in the spread of these antibiotic-resistant bacteria.
Despite the clear threat that carbapenem-resistant K. pneumoniae pose to patients, more effective infection control in hospitals, including consideration of how patients move between hospitals and hygiene interventions, will have an impact.
Professor Hajo Grundmann, co-lead author and Head of the Institute for Infection Prevention and Hospital Hygiene at the Medical Centre, University of Freiburg, said: “We are optimistic that with good hospital hygiene, which includes early identification and isolation of patients carrying these bacteria, we can not only delay the spread of these pathogens, but also successfully control them. This research emphasises the importance of infection control and ongoing genomic surveillance of antibiotic-resistant bacteria to ensure we detect new resistant strains early and act to combat the spread of antibiotic resistance.”
The second survey of the Enterobacteriaceae bacteria family across hospitals in Europe is currently being planned. The data generated is made available through MicroReact4, a publicly-available, web-based tool developed by the Centre for Genomic Pathogen Surveillance. MicroReact will help researchers and healthcare systems to chart the spread of antibiotic resistance in pathogens like K. pneumoniae and monitor how they are evolving.
Professor David Aanensen, co-lead author and Director of the Centre for Genomic Pathogen Surveillance, said: “Genomic surveillance will be key to tackling the new breeds of antibiotic-resistant pathogen strains that this study has identified. Currently, new strains are evolving almost as fast as we can sequence them. The goal to establish a robust network of genome sequencing hubs will allow healthcare systems to much more quickly track the spread of these bacteria and how they’re evolving.”
References
2 The survey is titled EuSCAPE (European Survey of Carbapenemase-Producing Enterobacteriaceae).
3 For information on the One Health initiative see http://www.euro.who.int/en/health-topics/disease-prevention/antimicrobial-resistance/about-amr/one-health
4 The data is freely available via MicroReact visualisation platform.
Source: Wellcome Sanger Institute
30.07.2019