Advances of vascular MRI at higher field strengths
For the fourth article in his series of articles for European Hospital, Professor Stefan Schönberg of the Institute of Clinical Radiology and Nuclear Medicine (IKRN), University Hospital Mannheim, Medical Faculty of Mannheim, University of Heidelberg, invited his colleague from Mannheim, Henrik J Michaely, and experts from the David Geffen School of Medicine at UCLA for a round-table discussion on:
Stefan G Ruehm, Kambiz Nael, Derek Lohan and Henrik J Michaely* describe impressive images that benefit patient treatment

Contrast-enhanced MR angiography (CEMRA) has evolved as an increasingly competitive diagnostic modality challenging both catheter angiography and CT angiography (CTA) for imaging of nearly all vascular territories. Over the recent years, improvements in gradient technology, pulse sequences, and postprocessing algorithms, combined with dramatic improvements in radiofrequency (RF) technology, have enabled the current status of CEMRA applications at 1.5T. Lately, whole-body 3T MRI systems have been introduced, with the promise of greatly improved signal-to-noise ratio (SNR) compared to 1.5T. With 3T imaging it appears feasible to obtain almost double the available SNR compared to 1.5T.
However, the move from 1.5T to 3T involves more than just increasing SNR: For some pulse sequences, many of which are now routine for imaging at 1.5T, there are substantial trade-offs at 3T such as so-called dielectric resonance effect, which can result in bands of signal loss on the MR image, particularly when RF intensive techniques (e.g. steady state free precession sequences) are employed.
High field imaging at 3T has proven to be particularly beneficial in combination with parallel imaging techniques. This technique allows for a reduction of MRI measurement times by using spatial information from multiple surface coil elements at the same time to substitute for the overall number of phase-encoding steps which determine the scan length. With the appropriate coil arrangement and receiver chain, it is possible to accelerate an acquisition manifold. In this context, a specific number called ‘acceleration factor’ characterises the increase in data collection speed. However, the penalty for the increased speed of parallel acquisition is a drop in SNR. Since there is more SNR available at 3T, it is possible to use higher acceleration factors at SNR values still adequate for diagnostic image quality. Therefore with identical acquisition times, images can be collected with higher spatial resolution or greater anatomical coverage compared to 1.5T.
Contrast-enhanced MRA is based on the use of T1-weighted fast spoiled 3-D gradient recalled-echo sequence in combination with the T1-shortening effect of gadolinium-based contrast agents. As an attractive feature of contrast-enhanced MRA at 3T the sensitivity to injected gadolinium agents is increased. This is based on an increase of the longitudinal relaxation time (T1) of background tissues with higher field strengths, which allows the use of smaller volumes of paramagnetic contrast agents.
With recent advances in scanner gradient performance, fast data acquisition times for isotropic three-dimensional (3-D) data sets for 576 matrix acquisitions have become possible enabling high spatial-resolution 3-D imaging during a comfortable breath-holding period.
An additional feature of MRI is the capability to generate temporally resolved 3-D images that display the first-pass transit of contrast through the vascular system. Time-resolved MRA can provide additional functional information and requires only very small doses of contrast. For many applications, in-plane resolution can be preserved while through-plane resolution is commonly traded for rapid temporal sampling.
Clinical Applications
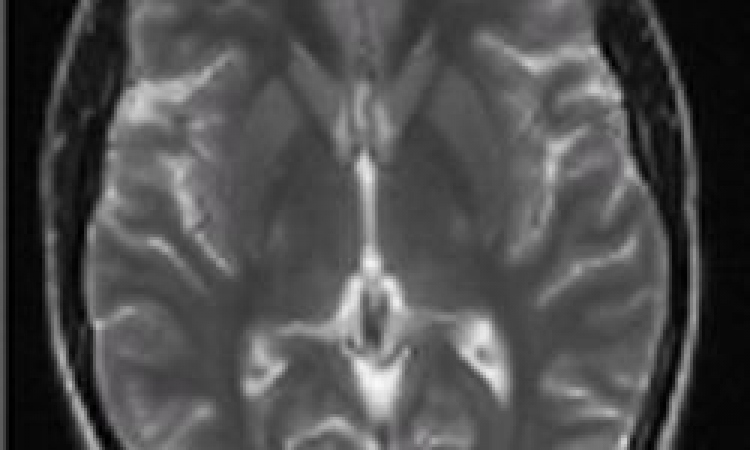
In many institutions 3-D CEMRA has found its role as the method of choice for the evaluation of the craniocervical vasculature.
Indications include a variety of conditions such as atherosclerotic disease, aneurysms, and arteriovenous malformations, presurgical assessment of tumours as well as post-treatment surveillance. The ability to time-resolved contrast-enhanced 3-D MRA with high spatial resolution is particularly valuable for the characterisation of arteriovenous malformations and allows the differentiation between high versus slow flow lesions. This may play an important role for determining adequate treatment strategies which include conservative treatment, embolisation therapy or surgery.
With a critical stenosis level of 70% as determined by the NASCET study to be the threshold for treatment of patients with thrombendarterectomy, improved spatial resolution for accurate stenosis grading of carotid arteries appears mandatory. Similarly, high spatial resolution imaging for improved characterisation of vascular wall invasion can play an important role to determine resectability of neck tumours. Craniocervical contrast-enhanced MRA can be used to evaluate patients following treatment for vascular disease, for example to assess re-stenosis after endovascular treatment following stent placement or for follow-up of patients with vessel dissection in order to detect possible progression of stenosis or occlusion which may mandate invasive therapy such as surgery or stenting to prevent ischemic cerebral complications.
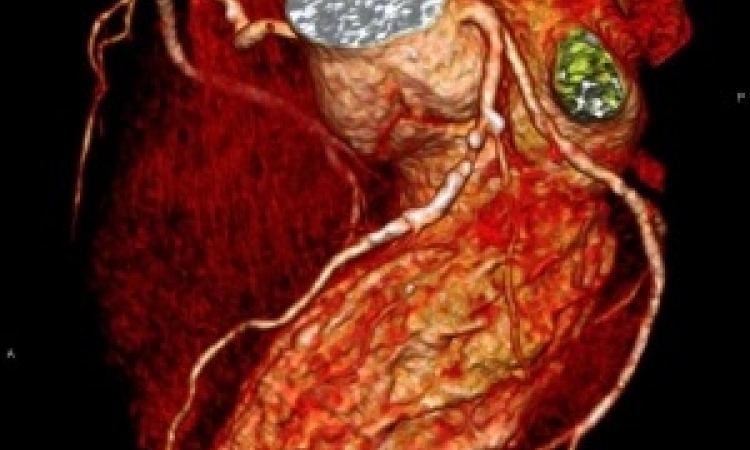
Applications in the pulmonary circulation include evaluation of pulmonary embolism, pulmonary hypertension, and congenital heart disease. Pulmonary venous MRA plays an increasing role for accurate planning and follow up of radiofrequency ablation therapy in patients with cardiac arrhythmia.
For the abdominal vasculature, clinical applications include the assessment of atherosclerotic arterial disease, aneurysms, and dissections, as well as the preoperative assessment of tumour extent. In addition, MR venography is a rapidly growing application in chest, abdomen, pelvis and lower extremity which benefits from high spatial resolution and the potential reduction of contrast volumes.
Indications for renal MRA include the diagnosis of atherosclerotic renal artery stenosis, fibromuscular dysplasia (FMD), renal aneurysms, dissections, as well as the evaluation of patients pre- and post renal transplantation. In combination with functional imaging techniques such as ultrasound, nuclear medicine imaging, phase-contrast MRA or perfusion MRI, the accurate characterisation and quantification of the severity of stenotic renal artery disease plays an important role for planning revascularisation strategies. High-resolution MRA at 3T may help to limit the over-estimation of the degree of stenotic disease and may therefore avoid unnecessary renal revascularisation procedures which may further increase the risk of patients with borderline renal function.
FMD represents the second most common cause of renal artery disease. It tends to affect younger patients. Patients benefit from early diagnosis since there is a good respond to balloon angioplasty. FMD usually affects the mid and distal artery segments. These segments may be missed on conventional MRA at 1.5T due to limited spatial resolution. It is expected that CEMRA at 3T increases sensitivity and specificity for the early detection of FMD.
Similarly to single station MRA, multistation peripheral or whole-body imaging can be performed at 3T yielding high spatial-resolution data sets with isotropic submillimeter voxel size. With the combination of parallel imaging, an appropriate contrast-injection protocol, and flexible table movement, venous contamination can be minimised or avoided. The procedure is feasible and holds promise for screening applications.
In summary, a wide spectrum of vascular diseases may benefit from imaging at 3T. Our experience suggests that CEMRA at 3T is robust and besides providing spectacular images holds promise to improve patient care by improved diagnostic accuracy which may positively affect therapeutic strategies.
*Henrik J Michaely is a consultant to BayerHealthCare
01.09.2008