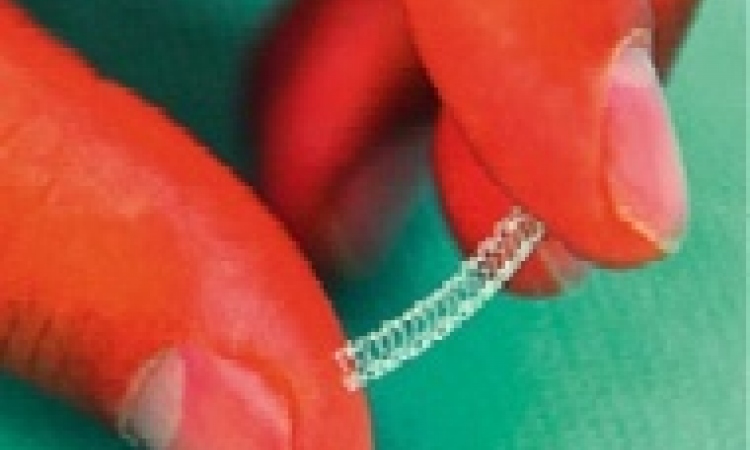
Transcatheter aortic valve implants
Although transcatheter aortic valve implantation (TAVI) is increasingly the most used surgical procedure in Germany, only two products have been approved for routine use. Although this has prompted other medical device manufacturers to go into action, according to Professor Justus Strauch, head of cardiac surgery at the Klinikum Bergmannsheil, Bochum, no one has yet taken the lead in this technology. Interview: Holger Zorn

The leap from the 157 TAVI procedures carried out in 2007 to the 3,629 undertaken in 2010 is impressive. Was it sely the German reimbursement policy – almost €35,000 per procedure – that boosted the use of this novel treatment for severe aortic valve stenosis?
Prof. Strauch: ‘Definitely not! Taking the results of the Helsinki Ageing Study, 2.9% of 75-86-year-olds suffer from this disease. In relation to the German population, this means 160,000 potential patients. A high proportion of these patients are too frail for conventional surgical valve replacement – and conservative treatment is of limited success. They were underserved, if not un-served. With minimally-invasive, catheter-based aortic valve replacement we can prolong their life expectancy and improve their life quality.’
Is there evidence of that?
‘Yes. The PARTNER trial -- with 358 patients at 21 centres the largest multicentre study on transcatheter aortic valve implantation for aortic stenosis in patients who cannot undergo surgery -- has shown a significantly reduced rate of death from any cause after one year. These data are confirmed by my own experience over the last four years.’
Those results were achieved with Edward’s Sapien valve, so far one of the two approved products. Meanwhile, other global players, as well as start-up companies, have announced a couple of new developments. Which one might win the race?
‘It’s really too early to say. One device, for example, from an Israeli start-up that was acquired two years ago by Medtronic, was completely withdrawn from all clinical evaluation. Some other companies -- even global players such as St. Jude Medical – have only reported the first human implant. Others are still undergoing feasibility trials and the most advanced start-ups, JenaValve Technology in Munich, or Symetis in Lausanne, have reported only series of less than 100 patients with short-term outcome data. So far, the Sapien valve with its transapical approach has a technological edge of two to three years with proven results.’
What do you expect from a next generation valve?
‘From a next generation valve I’d want the property of re-positioning within the procedure, a reduced rate of paravalvular leakage and a smaller dimensioned introducer sheath.’
26.08.2011