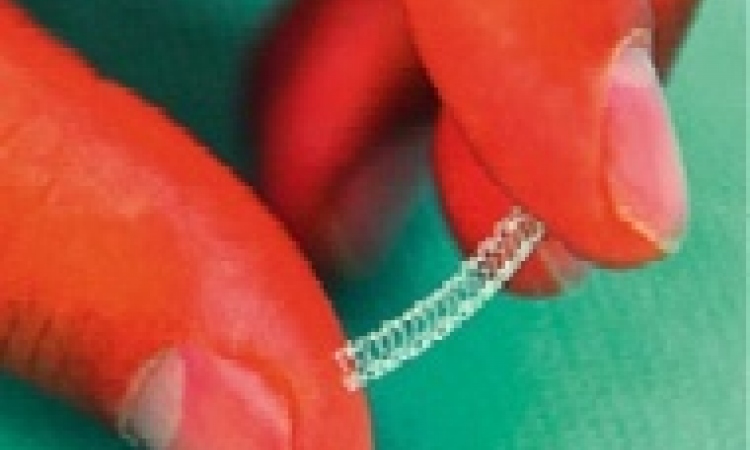
Platinum chromium alloy enhances design
Boston Scientific Corporation has commenced enrolment of a targeted 1,500 patients for the Taxus Perseus clinical trials, planned to take place in 100 international centres.

The aim is to evaluate the firm’s third-generation paclitaxel-eluting coronary stent - the Taxus Element Stent. This stent features the proprietary Platinum Chromium Alloy (designed specifically for stents) which, combined with a new stent design, is designed to allow thinner struts, increased flexibility, and a lower profile, while improving radial strength, recoil, and radiopacity, Boston Scientific reports, adding that the stent’s platform incorporates new balloon technology, intended to improve on the firm’s Maverick Balloon Catheter technology.
Dean J Kereiakes MD is principal investigator for the trials and Medical Director at The Christ Hospital Heart and Vascular Centre and The Lindner Research Centre, in Cincinnati. Louis A Cannon MD, of the Cardiac and Vascular Research Centre of Northern Michigan in Petoskey, is co-principal investigator.
Dr Kereiakes predicted: ‘This new platform, designed for improved deliverability, should allow us to bring the long-term proven performance of the Taxus Stent to even the most complex and challenging anatomy.’
The first of the two-part study, called Taxus Perseus Workhorse, will evaluate the safety and efficacy of the TAXUS Element Stent compared with Boston Scientific’s first generation drug-eluting stent (Taxus Express2). 1,264 patients with ‘workhorse’ lesions from 2.75 to 4.0 millimetres will be evaluated, with the primary endpoint of target lesion failure (TLF) at 12 months; its secondary endpoint is in-segment percent diameter stenosis at nine months.
The second part, the Taxus Perseus Small Vessel study, will compare the Taxus Element Stent with a historic control (the Taxus V de novo bare-metal Express Coronary Stent System). It will involve 224 patients with lesions from 2.25 up to 2.75 millimetres. The primary endpoint of the small vessel study is in-stent late loss at nine months, and its secondary endpoint is TLF at 12 months. Study success is dependent on both endpoints, Boston Scientific explains.
Hank Kucheman, Senior Vice President and Group President, Interventional Cardiology, said: ‘The platinum chromium alloy and new balloon technologies in this system are also being developed in an Everolimus version and is intended to serve as foundational technology in Boston Scientific’s dual-drug DES portfolio, including a drug-eluting bifurcation stent and next-generation Everolimus- and Paclitaxel-eluting stents.’
01.09.2007