Eye health
FDA Approves biosimilar to treat macular degeneration disease
The U.S. Food and Drug Administration approved Byooviz (ranibizumab-nuna) as the first biosimilar to Lucentis (ranibizumab injection) for the treatment of several eye diseases and conditions, including neovascular (wet) age-related macular degeneration (nAMD), a leading cause of vision loss and blindness for Americans aged 65 years and older.
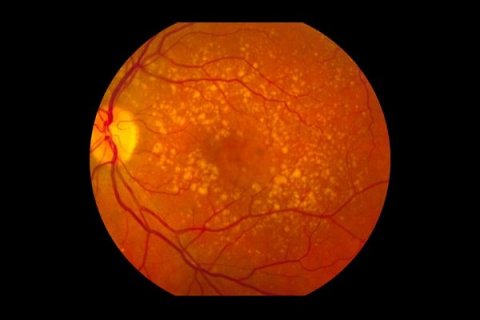
Image source: Intermediate age related macular degeneration, marked as public domain, more details on Wikimedia Commons
Byooviz is also approved to treat macular edema (fluid build-up) following retinal vein occlusion (blockage of veins in the retina) and myopic choroidal neovascularization, a vision-threatening complication of myopia (nearsightedness).
“Today’s approval provides another treatment option for millions of people whose vision is impaired and is another step forward in our commitment to provide access to safe, effective and high-quality biological products,” said Sarah Yim, M.D., director of the Office of Therapeutic Biologics and Biosimilars in the FDA’s Center for Drug Evaluation and Research. “Continuing to grow the number of biosimilar approvals is a key part of our efforts to provide greater access to treatment options for patients, increase competition and potentially lower costs.”
Biological products are generally derived from a living organism and can come from many sources, including animals and microorganisms, such as bacteria or yeast. A biosimilar is a biological product that is approved based on data showing that it is highly similar to a biological product already approved by the FDA (reference product) and has no clinically meaningful differences in terms of safety, purity and potency (i.e., safety and effectiveness) from the reference product, in addition to meeting other criteria specified by law. Patients can expect the same safety and effectiveness from the biosimilar over the course of treatment as from the reference product.
21.09.2021