News • FDA launches off-label drug database
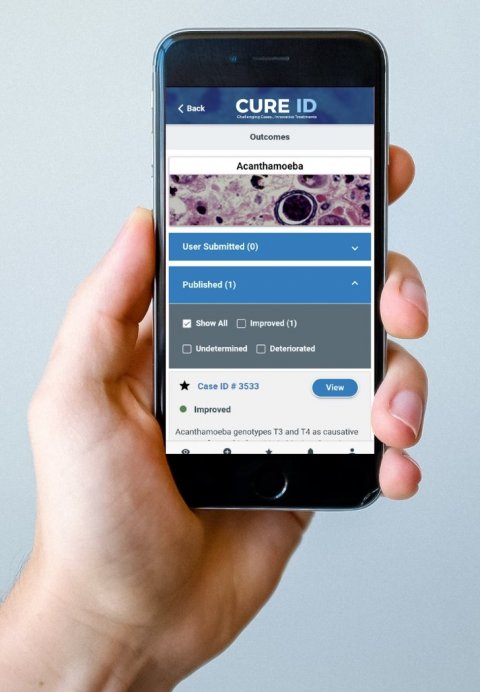
Cure ID: App finds new ways of treatment
The U.S. Food and Drug Administration (FDA) announced the global launch of Cure ID, an internet-based repository that will allow the clinical community to report their experiences treating difficult-to-treat infectious diseases with novel uses of existing FDA-approved drugs through a website, a smartphone or other mobile device.
The platform enables the crowdsourcing of medical information from health care providers to guide potentially life-saving interventions and facilitate the development of new drugs for neglected diseases. The repository is a collaboration between the FDA and the National Center for Advancing Translational Sciences (NCATS), which is part of the National Institutes of Health (NIH).
“The Cure ID application focuses on drugs for infectious diseases lacking adequate treatments, including neglected tropical diseases, emerging infectious threats and infections caused by antimicrobial-resistant organisms. When health care professionals directly input their clinical cases into the app, Cure ID allows these real-world experiences to be organized and analyzed much faster, making it easier to spot promising new uses for existing drugs,” said Amy Abernethy, M.D., Ph.D., FDA Principal Deputy Commissioner. “Our hope is that this app will serve as a connector among major treatment centers, academics, private practitioners, government facilities and other health care professionals from around the world and ultimately get treatments to patients faster.”
The repository captures clinical outcomes when drugs are used for new indications, in new populations, in new doses or in new combinations. Health care professionals generally may choose to prescribe or use a legally marketed human drug or medical device for an unapproved or uncleared use when they judge that the unapproved use is medically appropriate for an individual patient. The systematic collection of real-world experience in the app will help identify drug candidates for additional study, encourage further drug development, and may serve as a resource for practitioners making individual patient treatment decisions in the absence of established safe and effective options. Repurposing approved drugs for new clinical indications can potentially offer an efficient drug-development pathway for treatments of diseases and conditions that have few or no therapeutic options.
The potential importance of new therapeutic opportunities from repurposing drugs can’t be understated
Christopher P. Austin
“The potential importance of new therapeutic opportunities from repurposing drugs can’t be understated,” said NCATS Director Christopher P. Austin, M.D. “The Cure ID platform exemplifies how collaborative efforts can spark innovations that benefit patients. This new platform harnesses the power of crowdsourcing to help gather medical observations in the field and help identify potentially effective treatments for diseases.”
The app works by collecting a simple case report form from caregivers about their experience using an approved product for an unapproved use. Health care professionals can browse from a collection of cases that have already been documented, including successful and unsuccessful treatments, in addition to viewing relevant clinical trials and those open to enrollment at clinicaltrials.gov. App users can also participate in a treatment discussion forum where they can engage with fellow providers globally. The FDA plans to reach out to health care providers in various disciplines, including infectious and tropical diseases, to encourage them to use the app.
The full launch of the app follows the release of several pilot versions after an initial innovation award was received from the U.S. Department of Health & Human Services IDEA Lab in April 2015. During the pilots, extensive user-testing was conducted in India (2015 and 2017) and South Africa (2016). Additional feedback was also collected from users in the U.S., Europe and Peru. The updated app that launched today includes the addition of a newsfeed, an improved search feature with data from 325 different infectious diseases and syndromes to choose from, and the inclusion of nearly 1,500 initial cases from clinicians and the published literature and over 18,000 clinical trials.
Source: U.S. Food and Drug Administration (FDA)
06.12.2019