Sponsored • Reprocessing equipment
A breakthrough technology for endoscope drying and storage
Due to their complex design, reusable endoscopes have been long recognized to require thorough reprocessing to properly disinfect. In practice, the drying of the endoscope is often underestimated. We speak to Dr. Daniel Vinteler, CEO and Founder of Plasmabiotics, about the importance of drying and the newest generation PlasmaTyphoon+ and PlasmaBag system, for faster complete endoscope drying and a more intuitive user experience. A breakthrough technology designed to improve endoscope reprocessing to enhance patient safety and hospital efficiency.
Why is it so crucial to completely dry an endoscope after the device has been disinfected?

Daniel Vinteler: “Every step of endoscope reprocessing is crucial to ensure a hygienic procedure: the pre-cleaning, cleaning disinfection, drying and storage. If one of these steps is not performed correctly, hygiene is not guaranteed. As failure of drying can result in growth of biofilms inside the endoscope during storage1, not only thorough cleaning, but also drying and storage become all the more important.2 In practice, we see that the drying of the endoscope is often underestimated.”
How do the PlasmaTyphoon and PlasmaTyphoon+ completely dry an endoscope?
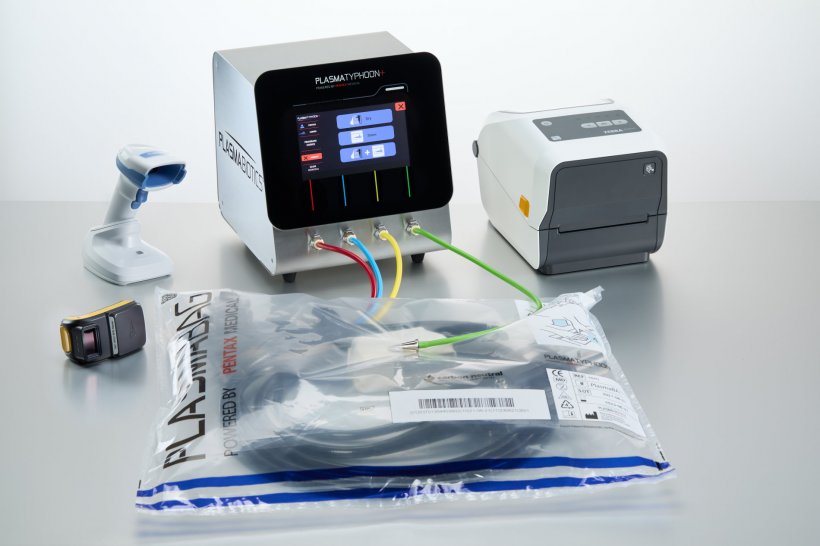
“The PlasmaTyphoon offers a new way to completely dry the scope, thereby reducing the risk of contamination. The drying process with the PlasmaTyphoon is managed by a patented curve of pressure. The unit uses a laminar flow to eliminate the water and a turbulent heated flow to dry the walls.”
What is the function of the PlasmaBag?
“After the completion of the drying process with the PlasmaTyphoon, the single use PlasmaBag comes into play: plasma, containing ozone molecules, is insufflated into the bag ensuring the dry and disinfected state of the endoscope is maintained. The PlasmaBag removes the need for additional reprocessing after storage and ensures the endoscopes are immediately available at the point of care, anywhere, anytime. The PlasmaBag prevents the risk of infection upon storage and transportation. We now also offer a carbon neutral PlasmaBag, offsetting all carbon emission emitted during the production process. Pentax Medical will soon take further steps to provide a further sustainability solution.”
What advantages can staff expect while using both the PlasmaTyphoon/PlasmaTyphoon+ and PlasmaBag system?
The small footprint required for endoscope drying and storage greatly reduces costs and space needed
Daniel Vinteler
“The advantages for staff are numerous. For example, the PlasmaTyphoon or PlasmaTyphoon+ and PlasmaBag system greatly increases reprocessing efficiency and improves endoscope turnover, thereby reducing wait times between procedures, allowing for more patient treatments. The small footprint required for endoscope drying and storage greatly reduces costs and space needed. Other advantages include the mobility of the scopes while they are stored in the PlasmaBag, and of course an improved level of hygiene.”
Who will benefit from the PlasmaTyphoon or PlasmaTyphoon+ and PlasmaBag system?
“Essentially the system can help you to increase hospital efficiency in every department in your hospital or clinic. The ICU has immediate access to ready-to-use endoscopes due to the unlimited drying capacity and storage for up to 31 days.3,4,5 The endoscopy unit can reduce waiting times between procedures thanks to the improved turnaround times and the reprocessing unit will experience more efficient workflows with seamless and fully traceable processes.”
What can you tell about the newest generation, the PlasmaTyphoon+?
“This month the newest generation of the PlasmaTyphoon system will be launched, the PlasmaTyphoon+.6 Both systems accelerate endoscope reprocessing by substantially reducing drying times from hours to just minutes, while maintaining the disinfected state of endoscopes for up to 31 days.7 The newest generation PlasmaTyphoon+ dries even faster than before, in 1-3 minutes, and offers a seamless user experience through the newly added intuitive touch panel and colour-coded tubes, making processes safer and easier for reprocessing staff. All the newly added elements are directly based on feedback from clinical users of the first generation PlasmaTyphoon and PlasmaBag system.”
Can you elaborate how the PlasmaTyphoon+ improves reprocessing processes on the hospital floor?
“The PlasmaTyphoon+ was designed to make handling routines simpler for reprocessing staff. The intuitive software guides the user through procedures on a touch screen interface. Additionally, processes are improved with visual guidance such as the new colour-coded tubing. The PlasmaTyphoon+ also offers full traceability both printed and digital with audit-ready data-records. This can be connected to hospital network data management systems to enable linkage of the drying and storage cycles and patient data. Finally, the PlasmaTyphoon+ has an automatic validation with integrated alarms. All these new elements were designed to provide additional support and safety in daily processes, as the system better assists reprocessing staff.”
For more information about the PlasmaTyphoon+ and PlasmaBag system, please visit: hygiene.pentaxmedical.com.
References:
- Robert Koch Institute (RKI). Recommendation of the commission for hospital hygiene and prevention of the RKI. Hygiene requirements for reprocessing flexible endoscopes and additional endoscopic instrumentation. Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz 2002;45:395 – 411
- Kovaleva J. Endoscope drying and its pitfalls. J Hosp Inf 2017:97;4:319 – 328
- Evaluation of the ability of a storage system (plasmabiotics) to maintain the microbiological quality of heat sensitive endoscope. Report by Biotech-Germande April 2017.
- Storage times are subject to local regulations.
- Validated for up to 744 storage hours (31 days) according to NF EN 16442 norm.The maximum storage time may be subject to local regulations on endoscope storage
- Limited release in 2021 with other markets to follow in 2022.
- Validated for up to 744 storage hours (31 days) according to NF EN 16442 norm.The maximum storage time may be subject to local regulations on endoscope storage
Source: Pentax Medical
16.12.2021